
THE KAPPE LABORATORY
Institute of Chemistry, University of Graz, Austria
Institute of Chemistry, University of Graz, Austria
C. Oliver Kappe is Professor of Chemistry at the University of Graz, Austria.

The Kappe Laboratories are housed at the Institute of Chemistry (IfC), University of Graz,
He received his diploma- (1989) and his doctoral (1992) degrees in organic chemistry from the University of Graz where he worked with Professor Gert Kollenz on cycloaddition and rearrangement reactions of acylketenes.
C. Oliver Kappe is Professor of Organic Chemistry and Director of the Christian Doppler Laboratory for Microwave Chemistry (CDLMC) at the University of Graz, Austria. He received his diploma- (1989) and his doctoral (1992) degrees in organic chemistry from the University of Graz where he worked with Professor Gert Kollenz on cycloaddition and rearrangement reactions of acylketenes. After periods of postdoctoral research work on reactive intermediates and matrix isolation spectroscopy with Professor Curt Wentrup at the University of Queensland in Brisbane, Australia (1993-1994) and on synthetic methodology/alkaloid synthesis with Professor Albert Padwa at Emory University in Atlanta, USA (1994-1996), he moved back to the University of Graz in 1996 to start his independent academic career. He obtained his "Habilitation" in 1998 in organic chemistry and was appointed Associate Professor in 1999. Since 2011 he holds the position of Professor of "Technology of Organic Synthesis" (Organische Synthesetechnologie) at the University of Graz. He has spent time as visiting scientist/professor at e.g. the Scripps Research Institute (La Jolla, USA, Professor K. Barry Sharpless, 2003), the Toyko Institute of Technology (Toyko, Japan, Professor T. Takahashi, 2008), the University of Sassari (Sassari, Italy, 2008), and the Sanford-Burnham Institute for Medical Research (Orlando, USA, 2010).
The co-author of ca. 300 publications, his main research interests have in the past focused on multicomponent reactions, combinatorial chemistry and the synthesis of biologically active heterocycles. More recently his research group has been involved with enabling technologies for synthetic chemistry, including microwave and continuous flow chemistry. For his innovative work in microwave chemistry he received the 2004 Prous Science Award from the European Federation for Medicinal Chemistry and the 2010 Houska Prize (100.000 €) in addition to a number of other awards.
DR SANJAY BAJAJ,,,,,,,,,,,,,,,,,,,DR ANTHONY CRASTO............PROF OLIVER KAPPE
FLOW CHEM CONFERENCE , MUMBAI, 22 JAN 2015......SELECTBIO
After periods of postdoctoral research work on reactive intermediates and matrix isolation spectroscopy with Professor Curt Wentrup at the University of Queensland in Brisbane, Australia (1993-1994) and on synthetic methodology/alkaloid synthesis with Professor Albert Padwa at Emory University in Atlanta, USA (1994-1996), he moved back to the University of Graz in 1996 to start his independent academic career. He obtained his "Habilitation" in 1998 in organic chemistry and was appointed Associate Professor in 1999. Since 2011 he holds the position of Professor of "Technology of Organic Synthesis" (Organische Synthesetechnologie) at the Instittue of Chemistry at the University of Graz. He has spent time as visiting scientist/professor at e.g. the Scripps Research Institute (La Jolla, USA, Professor K. Barry Sharpless, 2003), the Toyko Institute of Technology (Toyko, Japan, Professor T. Takahashi, 2008), the University of Sassari (Sassari, Italy, 2008), the Sanford-Burnham Institute for Medical Research (Orlando, USA, 2010) and the Federal University of Rio de Janeiro (Ri de Janeiro, Brazil, 2013).
The Kappe Laboratories are housed at the Institute of Chemistry (IfC), University of Graz,
He received his diploma- (1989) and his doctoral (1992) degrees in organic chemistry from the University of Graz where he worked with Professor Gert Kollenz on cycloaddition and rearrangement reactions of acylketenes.
C. Oliver Kappe is Professor of Organic Chemistry and Director of the Christian Doppler Laboratory for Microwave Chemistry (CDLMC) at the University of Graz, Austria. He received his diploma- (1989) and his doctoral (1992) degrees in organic chemistry from the University of Graz where he worked with Professor Gert Kollenz on cycloaddition and rearrangement reactions of acylketenes. After periods of postdoctoral research work on reactive intermediates and matrix isolation spectroscopy with Professor Curt Wentrup at the University of Queensland in Brisbane, Australia (1993-1994) and on synthetic methodology/alkaloid synthesis with Professor Albert Padwa at Emory University in Atlanta, USA (1994-1996), he moved back to the University of Graz in 1996 to start his independent academic career. He obtained his "Habilitation" in 1998 in organic chemistry and was appointed Associate Professor in 1999. Since 2011 he holds the position of Professor of "Technology of Organic Synthesis" (Organische Synthesetechnologie) at the University of Graz. He has spent time as visiting scientist/professor at e.g. the Scripps Research Institute (La Jolla, USA, Professor K. Barry Sharpless, 2003), the Toyko Institute of Technology (Toyko, Japan, Professor T. Takahashi, 2008), the University of Sassari (Sassari, Italy, 2008), and the Sanford-Burnham Institute for Medical Research (Orlando, USA, 2010).
The co-author of ca. 300 publications, his main research interests have in the past focused on multicomponent reactions, combinatorial chemistry and the synthesis of biologically active heterocycles. More recently his research group has been involved with enabling technologies for synthetic chemistry, including microwave and continuous flow chemistry. For his innovative work in microwave chemistry he received the 2004 Prous Science Award from the European Federation for Medicinal Chemistry and the 2010 Houska Prize (100.000 €) in addition to a number of other awards.
DR SANJAY BAJAJ,,,,,,,,,,,,,,,,,,,DR ANTHONY CRASTO............PROF OLIVER KAPPE
FLOW CHEM CONFERENCE , MUMBAI, 22 JAN 2015......SELECTBIO
After periods of postdoctoral research work on reactive intermediates and matrix isolation spectroscopy with Professor Curt Wentrup at the University of Queensland in Brisbane, Australia (1993-1994) and on synthetic methodology/alkaloid synthesis with Professor Albert Padwa at Emory University in Atlanta, USA (1994-1996), he moved back to the University of Graz in 1996 to start his independent academic career. He obtained his "Habilitation" in 1998 in organic chemistry and was appointed Associate Professor in 1999. Since 2011 he holds the position of Professor of "Technology of Organic Synthesis" (Organische Synthesetechnologie) at the Instittue of Chemistry at the University of Graz. He has spent time as visiting scientist/professor at e.g. the Scripps Research Institute (La Jolla, USA, Professor K. Barry Sharpless, 2003), the Toyko Institute of Technology (Toyko, Japan, Professor T. Takahashi, 2008), the University of Sassari (Sassari, Italy, 2008), the Sanford-Burnham Institute for Medical Research (Orlando, USA, 2010) and the Federal University of Rio de Janeiro (Ri de Janeiro, Brazil, 2013).
The co-author of ca. 350 publications, his main research interests have in the past focused on multicomponent reactions, combinatorial chemistry and the synthesis of biologically active heterocycles. More recently his research group has been involved with enabling and process intensification technologies, including microwave and continuous flow chemistry. For his innovative work in microwave chemistry he received the 2004 Prous Science Award from the European Federation for Medicinal Chemistry and the 2010 Houska Prize (100.000 €) in addition to a number of other awards.

C. Oliver Kappe is currently Editor-in-Chief of the Journal of Flow Chemistry (Akadémiai Kiadó) and a board member of the Flow Chemistry Society. In addition he has been an Editor of the Journal QSAR and Combinatorial Sciences (Wiley-VCH, 2003-2007) and has served/serves on the Editorial/Advisory Boards of the Journal of Combinatorial Chemistry (ACS), Molecular Diversity (Springer), ChemMedChem and ChemSusChem (Wiley-VCH), Journal of Heterocyclic Chemistry (Wiley-VCH) and a number of other journals.
Publications
Prof. C. O. Kappe has co-authored
ca. 350 scientific publications since 1988 in a variety of different
fields, including review articles, books and book chapters. For an
overview of publications and citations, see: Publications/Citations from ResearchID.com. His current H-index is 58 (September 2014).
He is most well-known for his work
in the field of microwave chemistry and his laboratory has authored over
150 original research articles, reviews and books on microwave-assisted
synthesis since 1999. He has contributed chapters to most of the
available reference books on the subject and has published numerous
other review and feature articles on microwave synthesis.
Most notably
among those is a 2004 review in Angewandte Chemie, which has
been voted best review article in this journal and is currently (year
2013) the most cited review in microwave synthesis. The comprehensive
books "Microwaves in Organic and Medicinal Chemistry" and "Practical Microwave Synthesis for Organic Chemists - Strategies, Instruments, and Protocols" were
published with Wiley-VCH in 2005 (2nd ed. 2012) and 2009, respectively
and are currently considered the standard reference books in the field.
Recent publications focus on continuous
flow chemistry, the use of microreactors in organic chemistry and
process intensification.
The work of the research team in Graz has also been highlighted in articles published in Chemical & Engineering News (C&EN, December 13, 2004 Issue; C&EN, October 12, 2009 Issue; and C&EN, September 24, 2012 Issue), Nature Magazine (Nature 2003, 421, 571-572; and Nature 2009, 461, 701) and in Chemistry World (Chemistry World 2008, 5, Issue 10, Chemistry World 2009, 6, Issue 11, Chemistry World 2013, June 19, Chemistry World 2013, July 22, Chemistry World 2013, October 22.
Recent Hot Papers from the Kappe Lab:
Microwave Effects in Organic Synthesis – Myth or Reality?
C. O. Kappe, B. Pieber, D. Dallinger,
Angew. Chem. Int. Ed. 2013, 52, 1088
C. O. Kappe, B. Pieber, D. Dallinger,
Angew. Chem. Int. Ed. 2013, 52, 1088
In Situ Generation of Diimide from Hydrazine and Oxygen - Transfer Hydrogenation of Olefins in Continuous Flow.
B. Pieber, S. T. Martinez, D. Cantillo, C. O. Kappe
Continuous Flow Generation and
Reactions of Anhydrous Diazomethane
Using a Teflon AF-2400 Tube-in-Tube
Reactor
F. Mastronardi, B. Gutmann, C. O. Kappe,
Org. Lett. 2013, 15, 5590
F. Mastronardi, B. Gutmann, C. O. Kappe,
Org. Lett. 2013, 15, 5590
Shifting Chemical Equilibria in Flow – Efficient Decarbonylation Chemistry Driven by Annular Flow Regimes.
B. Gutmann, P. Elsner, T. Glasnov, D. M. Roberge, C. O. Kappe
Use of Continuous Flow Technology to
Harness Hazardous Chemistries and Process Conditions – A Tool for the
Manufacturing of Active Pharmaceutical Ingredients (Review)
B. Gutmann, D. Cantillo, C. O. Kappe
Angew. Chem. Int. Ed. 2015, 54, in press.
The Kappe Lab in the Press:
New Stirring Design for Microwaves
Microwave Quarrel Heats Up
Diazomethane without Tears. Or Explosions
An Interview with C. O. Kappe
Microwave Effects Debate
.......................................
new article
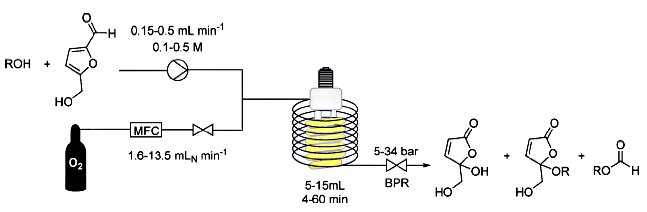
C. Oliver Kappe, University of Graz, Austria, and colleagues prepared for the first time the potential new platform molecule H2MF in pure form and converted it to the polyester precursor 5-hydroxy-4-keto-pentenoic acid (HKPA).
read at
Research
Research in the Kappe group focuses
on enabling technologies in organic synthesis and related areas. Of
primary current interest is the use of microwave dielectric heating and continuous flow/microreactor technology.
We are particularly interested to improve existing and/or develop new
synthetic procedures (in many cases of significant industrial interest)
using both of these technologies, either separately, or in combination
with each other. Emphasis is also placed on the sustainability of the
developed procedures.
FLOW CHEMISTRY
The use of microreactors and
contnuous flow equipment in general has opened up new horizons for
synthetic organic chemistry and the chemical manufacturing industry.
Microreaction technology is generally defined as the continuous flow
processing of reactions within structured channels of 10-500 micrometer
diameter. Because of the high surface-to-volume ratio in microchannels
of this type, heat transfer is very efficient and reaction
temperatures in microreactors can be changed efficiently by application
or removal of heat. In addition, enhanced mass transfer
characteristics, safer synthesis of dangerous compounds, isolation of
air and moisture sensitive chemistry, and reduction of hazardous waste
can all be realized using microreactors. The ability to efficiently
optimize reaction conditions by control of residence time and rapid
experimentation also add value to the technology by shortening
production development lifecycles. A particularly attractive feature of
microreaction technology is the ease with which reaction conditions
can be scaled - without the need for reoptimization - through the
operation of multiple systems in parallel (numbering-up, scaling-out),
thereby achieving production scale capabilities.
Emphasis in our work in the area of
flow chemistry is placed on process intensification techniques, in
particular working in high-temperature/high-pressure environments (Novel
Process Windows), often dealing with extremely hazardous chemical
transformations. We also have an interest in working with multiphasic
(gas/liquid, gas/liquid/solid) flow regimes.
Recent published examples of our flow chemistry projects are highlighted below:
Continuous Flow Microreactor Chemistry Under High Temperature/Pressure Conditions.
T. Razzaq, T. N. Glasnov, C. O. Kappe, Eur. J. Org. Chem. 2009, 1321-1325.
Translating High-Temperature Microwave Chemistry to Scalable Continuous Flow Processes.T. Razzaq, T. N. Glasnov, C. O. Kappe, Eur. J. Org. Chem. 2009, 1321-1325.
M. Damm, T. N. Glasnov, C. O. Kappe, Org. Process Res. Develop. 2010, 14, 215-224.
The Microwave-to-Flow Paradigm: Translating High-Temperature Batch Microwave Chemistry to Scalable Continuous Flow Processes.
T. N. Glasnov, C. O. Kappe, Chem. Eur. J. 2011, 17, 11956-11968.
T. N. Glasnov, C. O. Kappe, Chem. Eur. J. 2011, 17, 11956-11968.
______________________________________________________________________________
Synthesis of 5-Substituted 1H-Tetrazoles from Nitriles and Hydrazoic Acid Using a Safe and Scalable High-Temperature Microreactor Approach.
B. Gutmann, J.-P. Roduit, D. Roberge, C. O. Kappe, Angew. Chem. Int. Ed. 2010, 49, 7101-7105.
Mechanistic Insights on Azide-Nitrile Cycloadditions: On the
Dialkyltin Oxide-Trimethylsilyl Azide Route and a New
Vilsmeier-Haack-Type Organocatalyst. B. Gutmann, J.-P. Roduit, D. Roberge, C. O. Kappe, Angew. Chem. Int. Ed. 2010, 49, 7101-7105.
D. Cantillo, B. Gutmann, C. O. Kappe, J. Am. Chem. Soc. 2011, 133, 4465-4475.
Safe Generation and Synthetic Utilization of Hydrazoic Acid in a Continuous Flow Reactor.
B. Gutmann, J.-P. Roduit, D. Roberge, C. O. Kappe, J. Flow Chem. 2012, 2,8-19.
______________________________________________________________________________
In Situ Generated Iron Oxide
Nanocrystals as Efficient and Selective Catalysts for the Reduction of
Nitroarenes in Continuous Flow.
D. Cantillo, M. Baghbanzadeh, C. O. Kappe, Angew. Chem. Int. Ed. 2012, 51, 10190-10193.
Hydrazine-Mediated Reduction of Nitro and Azide Functionalities Catalyzed by Highly Active and Reusable Magnetic Iron Oxide Nanocrystals.
D. Cantillo, M. Mirhosseini Moghaddam, C. O. Kappe, J. Org. Chem. 2013, 78, 4530-4542.
Immobilized Iron Oxide Nanoparticles as Stable and Reusable Catalysts for Hydrazine-mediated Nitro Reductions in Continuous Flow
M. Mirhosseini Moghaddam, B. Pieber, T. Glasnov, C. O. Kappe,
ChemSusChem 2014, 7, in press.
______________________________________________________________________________
Methylation Using Dimethylcarbonate Catalysed by Ionic Liquids Under Continuous Flow Conditions.
T. N. Glasnov, J. D. Holbrey, C. O. Kappe, K. R. Seddon, T. Yan, Green Chem. 2012, 14, 3071-3076.
______________________________________________________________________________
Direct Aerobic Oxidation of 2-Benzylpyridines in a Gas-Liquid Continuous-Flow Regime Using Propylene Carbonate as Solvent.
B. Pieber, C. O. Kappe, Green Chem. 2013, 15, 320-324.
______________________________________________________________________________
Continuous Flow Synthesis of Adipic Acid from Cyclohexene Using Hydrogen Peroxide in High-Temperature Explosive Regimes.
M. Damm, B. Gutmann, C. O. Kappe, ChemSusChem 2013, 6, 978-982.
______________________________________________________________________________
In Situ Generation of Diimide from Hydrazine and Oxygen - Transfer Hydrogenation of Olefins in Continuous Flow.
B. Pieber, S. T. Martinez, D. Cantillo, C. O. Kappe, Angew. Chem. Int. Ed. 2013, 52, 10241.
______________________________________________________________________________
A Three Step Continuous Flow Synthesis of the Biaryl Unit of the HIV Protease Inhibitor Atazanavir.
L. Dalla-Vechia, B. Reichart, T. N. Glasnov, L. S. M. Miranda, C. O. Kappe, R. O. M. A. de Souza,
Org. Biomol. Chem. 2013, 11, 6806.
Continuous Flow Synthesis of alpha-Haloketones – Essential Building Blocks of Antiretroviral Agents.
V. D. Pinho, B. Gutmann, L. S. M. Miranda, R. O. M. A. de Souza, C. O. Kappe,
J. Org. Chem. 2014, 79, in press.
______________________________________________________________________________
Continuous Flow Generation and Reactions of Anhydrous Diazomethane Using a Teflon AF-2400 Tube-in-Tube Reactor.
F. Mastronardi, B. Gutmann, C. O. Kappe,
Org. Lett. 2013, 16, 5590-5593.
Continuous Flow Synthesis of alpha-Haloketones – Essential Building Blocks of Antiretroviral Agents.
V. D. Pinho, B. Gutmann, L. S. M. Miranda, R. O. M. A. de Souza, C. O. Kappe,
J. Org. Chem. 2014, 79, 1555-1562
______________________________________________________________________________
A Scalable Procedure for Light Induced Benzylic Brominations in Continuous Flow.
D. Cantillo, O. de Frutos, J. A. Rincon, C. Mateos , C. O. Kappe,
J. Org. Chem. 2014, 79,223-229.
Continuous Flow alpha-Trifluoromethylation of Ketones by Metal Free Visible Light Photoredox Catalysis.
D. Cantillo, O. de Frutos, J. A. Rincon, C. Mateos , C. O. Kappe,
Org. Lett. 2013, 17, 5590-5593.
______________________________________________________________________________
Flash Carboxylation: Fast Lithiation - Carboxylation Sequence at Room Temperature in Continuous Flow.
B. Pieber, T. Glasnov, C. O. Kappe,
RSC Adv. 2014, 4, 13430-13433.
______________________________________________________________________________
Continuous Flow Synthesis of alpha-Haloketones – Essential Building Blocks of Antiretroviral Agents.
V. D. Pinho, B. Gutmann, L. S. M. Miranda, R. O. M. A. de Souza, C. O. Kappe,
J. Org. Chem. 2014, 79, 1555-1562.
______________________________________________________________________________
D. Cantillo, M. Damm, D. Dallinger, M. Bauser, M. Berger, C. O. Kappe
Org. Process Res. Develop. 2014, 18, in press.
______________________________________________________________________________
Shifting Chemical Equilibria in Flow – Efficient Decarbonylation Chemistry Driven by Annular Flow Regimes.
B. Gutmann, P. Elsner, T. Glasnov, D. M. Roberge, C. O. Kappe,
Angew. Chem. Int. Ed. 2014, 53, in press.
B. Gutmann, P. Elsner, T. Glasnov, D. M. Roberge, C. O. Kappe,
Angew. Chem. Int. Ed. 2014, 53, in press.
______________________________________________________________________________
Microwave Chemistry
The group has a 15 year experience in
microwave chemistry in a variety of different disciplines, including
organic synthesis, solid-phase peptide chemistry, the generation of
nanomaterials, and proteomics applications. Since 1999 more than 150
publications including several review articles and books were published.
We are able to perform microwave-assisted reactions from the
microliter scale using microtiter plates up to the liter scale employing
suitable large scale equipment.
Of particular interest over the past
years was the investigation of so-called microwave effects where we have
published extensively. For recent reviews on this topic see the
following references and follow this link:
Microwave Effects in Organic Synthesis – Myth or Reality?C. O. Kappe, B. Pieber, D. Dallinger, Angew. Chem. Int. Ed. 2013, 52, 1088-1094
Unraveling the Mysteries of Microwave Chemistry Using Silicon Carbide Reactor Technology.
C.O. Kappe, Acc. Chem. Res. 2013, 46, 1579-1585.
How to Measure Reaction Temperature in Microwave-heated Transformations.
C.O. Kappe, Chem. Sov. Rev. 2013, 42, 4977-4990.
....................
Microwave-Assisted Organic Synthesis in Near-Critical Water at 300 oC. A Proof-of-Concept Study
Jennifer M. Kremsner and C. Oliver Kappe
Karl-Franzens-University Graz
Jennifer M. Kremsner and C. Oliver Kappe
Karl-Franzens-University Graz
.........................
...................
C. Oliver Kappe of the Karl-Franzens-University, Graz has found (J. Org. Chem. 2007, 72, 4440. DOI: 10.1021/jo070408f) that thioamides such as 14 couple smoothly under Pd catalysis with areneboronic acids, even more rapidly than the usually reactive alkenyl bromide. Hans-Dieter Arndt of the Universität Dortmund has developed (J. Org. Chem. 2007, 72, 4205. DOI: 10.1021/jo0703505) a hetero Diels-Alder approach to pyridines, based on the addition of alkynes such as 16 to the diene 17. Richmond Sarpong of the University of California, Berkeley, has devised (Org. Lett. 2007, 9, 2167. DOI: 10.1021/ol070658i) an elegant Pt-catalyzed rearrangement of alkynyl aziridines such as 19, giving, after sulfinate elimination, pyridines such as 20.
.............................................................
Communication to me from a great scientist...cut paste below
 to ....................
to ....................
On fri 26 dec 2014
Dear Anthony,
a very pleasant surprise……Keep
up the good work.
Regards, oliver
Prof. C. Oliver Kappe
Institute of Chemistry,
University of Graz
Heinrichstrasse 28, A-8010 Graz,
Austria
Tel.: +43 316 3805352. Fax: +43
316 3809840
Email: oliver.kappe@uni-graz.at
Editor-in-Chief, Journal of Flow
Chemistry
.......................................................................................
GRAZ AUSTRIA

No comments:
Post a Comment