Dhananjay Sathe
Vice President, chemical research department at Unichem Laboratories Limited

Contact
in.linkedin.com/pub/dhananjay-sathe/18/34a/412
https://twitter.com/dgsathe, @dgsathe
https://www.facebook.com/dhananjay.sathe.35
http://www.unichemlabs.com/index.html
Vice President, chemical research department at Unichem Laboratories Limited
Contact
in.linkedin.com/pub/dhananjay-sathe/18/34a/412
https://twitter.com/dgsathe, @dgsathehttps://www.facebook.com/dhananjay.sathe.35
http://www.unichemlabs.com/index.html
Experience
Vice President, Research & Technology Development
Unichem Laboratories Limited
Guide API process research, Analytical Method Development, IPR groups. New project selection, exploring and implementing new technologies....................http://www.unichemlabs.com/index.html
Over the years, Unichem has invested significantly in its Research & Development (R&D) program to create state-of-the-art R&D facilities. Backed by a research team of over 150 highly-trained scientists with excellent credentials, the company’s R&D facilities are equipped with cutting-edge pharmaceutical research technology.
Guide API process research, Analytical Method Development, IPR groups. New project selection, exploring and implementing new technologies....................http://www.unichemlabs.com/index.html
Over the years, Unichem has invested significantly in its Research & Development (R&D) program to create state-of-the-art R&D facilities. Backed by a research team of over 150 highly-trained scientists with excellent credentials, the company’s R&D facilities are equipped with cutting-edge pharmaceutical research technology.
Over the years, Unichem has invested significantly in its Research & Development (R&D) program to create state-of-the-art R&D facilities. Backed by a research team of over 150 highly-trained scientists with excellent credentials, the company’s R&D facilities are equipped with cutting-edge pharmaceutical research technology.
Unichem’s laboratories are equipped with the latest sophisticated analytical instruments like XRD, NMR, GC, LC MSMS, IR/UV, TGA, DSC, Prep HPLC and Flash Chromatography among others.
The company’s expanded R & D facilities have strengthened its Chemical and Formulation capabilities. At the same time, its new Biotechnology R&D facility, which focuses on cost-effective bio-similar products using recombinant micro organisms, is geared to provide a new edge to Unichem’s Research & Development efforts.
Unichem’s laboratories are equipped with the latest sophisticated analytical instruments like XRD, NMR, GC, LC MSMS, IR/UV, TGA, DSC, Prep HPLC and Flash Chromatography among others.
The company’s expanded R & D facilities have strengthened its Chemical and Formulation capabilities. At the same time, its new Biotechnology R&D facility, which focuses on cost-effective bio-similar products using recombinant micro organisms, is geared to provide a new edge to Unichem’s Research & Development efforts.
Unichem’s R&D activities are focused on the following:
- Novel cost-efficient Process Developments for Active Pharmaceutical Ingredients (APIs) and intermediates
- Formulation Developments for generic APIs
- Non-infringing routes for manufacturing products to be marketed internationally
- Bio-similar products by recombinant DNA
- Contract research to develop formulations for New Chemical Entities (NCEs) and Generic APIs
Inspired by an ambition to drive path-breaking innovative research, Unichem aims to enhance people’s health by consistently providing better, more effective and safer products.
The Active Pharmaceutical Ingredient (API) business requires strong infrastructure, technological expertise and the experience and capability to carry out contract manufacturing and custom synthesis.
Unichem provides customers a complete solution for API development starting from samples and trial quantities right up to regulatory assistance that is required for registration and regulatory compliance.
The company’s facilities are designed to meet the highest global standards for API development with a focus on complex APIs, controlled substances and APIs for regulated markets, all of which are key to achieving the Unichem’s strategic goals.
The Active Pharmaceutical Ingredient (API) business requires strong infrastructure, technological expertise and the experience and capability to carry out contract manufacturing and custom synthesis.
Unichem provides customers a complete solution for API development starting from samples and trial quantities right up to regulatory assistance that is required for registration and regulatory compliance.
The company’s facilities are designed to meet the highest global standards for API development with a focus on complex APIs, controlled substances and APIs for regulated markets, all of which are key to achieving the Unichem’s strategic goals.
Associate Vice President
– (8 years 3 months)
Developed more than 80 APIs, including complex peptides and polymers. Set up preparative chromatography, TFF technologies.
– (8 years 3 months)
Developed more than 80 APIs, including complex peptides and polymers. Set up preparative chromatography, TFF technologies.
Sr. R&D Manager
RPG LifeSciences Ltd.
– (9 years)
Developed about 25 processes for APIs, intermediates, agrochemicals

– (9 years)

Developed about 25 processes for APIs, intermediates, agrochemicals

Education

Institute of Chemical Technology
M. Pharm, Pharmaceutical Chemistry
University of Mumbai
Ph.D, Pharmaceutical Chemistry
Poona College of Pharmacy
B. Pharm., Pharmaceutical Sciences
Courses
Institute of Chemical Technology

| Address |
|
| Phone | 022 2414 5616 |
| Website | http://www.ictmumbai.edu.in/ |
Institute of Chemical Technology , formerly the University Department of Chemical Technology , is a premier chemical engineering research institute located in Mumbai, Maharashtra,India. It is focused on training and research in various branches of chemical engineering, chemical technology, and pharmacy
Stereochemistry, Structure elucidation








GOA INDIA





 ANJUNA
ANJUNA

RAVE


SEASGOA MINE







 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
 Patents by Dhananjay Sathe
Patents by Dhananjay Sathe
Process for Preparation of Sevelamer Carbonate
Novel process for the preparation of 1-(9h-carbazol-4-yloxy)-3-[[2-(-methoxyphenoxy)-ethyl] amino]-propan-2-ol
Donepezil Hydrochloride Form VI
Process for preparing 5,6-dihydro-4-(S)-(ethylamino)-6-(S) methyl-4H-thieno[2,3b]thiopyran-2-sulphonamide-7,7-dioxide HCI
Novel process for preparation of clopidogrel bisulfate polymorph - Form I
Polymorph of (1-benzyl-4-[(5,6-dimethoxy-1-indanone)-2-y1] methyl piperidine hydrochloride (Donepezil hydrochloride) and a process for producing thereof
Process for the preparation of 4-(2-dipropylaminoethyl)-1,3-dihydro-2H-indol-2-one hydrochloride
Novel pharmaceutical salt of (1-benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine (Donepezil)
Patent Number Title Of Patent Date Issued 8187634 Process for the preparation of sevelamer hydrochloride and formulation thereof May 29, 2012 Disclosed herein is an improved process for preparation of Sevelamer hydrochloride having phosphate binding capacity of 4.7 to 6.4 mmol/g. Further, the invention discloses Sevelamer hydrochloride compositions and a novel process for preparation of said compositions comprising high sh 7847112 Polymorphs of atovaquone and process of preparation thereof December 7, 2010 Novel crystalline forms of anti Pneumocystis carinii compound (2-[4-(4-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone) commonly known as Atovaquone and methods for producing the same is disclosed herein. This also provides pharmaceutical compositions comprising the said polymo 7446200 Rapid resolution process of clopidogrel base and a process for preparation of clopidogrel bisulf November 4, 2008 The present invention discloses a rapid resolution process of racemic clopidogrel base followed by conversion of the resolved (S) isomer to crystalline Clopidogrel bisulfate Form I. The invention also discloses novel racemization process of the unwanted (R) isomer of clopidogrel base 7439365 Pharmaceutical salt of (1-benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine (Donepezi October 21, 2008 The present invention relates to the oxalate salt of 1-benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl]methyl piperidine, commonly known as Donepezil, and its method of preparation. 7378439 Process for the preparation of 4-(2-dipropylaminoethyl)-1,3-dihydro-2H-indol-2-one hydrochloride May 27, 2008 The present invention discloses a novel process and novel intermediates for the Preparation of 4-[2-(di-n-propyl amino) ethyl]-1,3-dihydro-2H-indol-2-one, commonly known as Ropinirole (I) and pharmaceutical composition comprising the same. Further the present invention also discloses 7186842 Polymorph of (1-benzyl-4-[(5,6-dimethoxy-1-indanone)-2-y1] methyl piperidine hydrochloride (Done March 6, 2007 The present invention discloses a novel, stable polymorph of 1-benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine hydrochloride commonly known as Donepezil hydrochloride. Further the present invention discloses a process for producing Donepezil HCl amorphous and its polymor

WO 2012025944
SITAGLIPTIN, SALTS AND POLYMORPHS THEREOFThe present invention relates to an improved process for preparation of Sitagliptin or pharmaceutically acceptable salts thereof. The present invention further relates to novel polymorphs of Sitagliptin salts and process for preparation thereof.
IPC: C07D487/04 applicants: AROTE NITIN DNYANESHWAR, SATHE DHANANJAY GOVIND, DAMLE SUBHASH VISHWANATH, USV LTD, AMBRE RAKESH RAMCHANDRA, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, Authors: AROTE NITIN DNYANESHWAR, DAMLE SUBHASH VISHWANATH, NAIK TUSHAR ANIL, AMBRE RAKESH RAMCHANDRA, SAWANT KAMLESH DIGAMBAR, SATHE DHANANJAY GOVIND, ...........http://www.google.co.in/patents/WO2012025944A2?cl=en

| Patent Number | Title Of Patent | Date Issued |
| 8187634 | Process for the preparation of sevelamer hydrochloride and formulation thereof | May 29, 2012 |
| Disclosed herein is an improved process for preparation of Sevelamer hydrochloride having phosphate binding capacity of 4.7 to 6.4 mmol/g. Further, the invention discloses Sevelamer hydrochloride compositions and a novel process for preparation of said compositions comprising high sh | ||
| 7847112 | Polymorphs of atovaquone and process of preparation thereof | December 7, 2010 |
| Novel crystalline forms of anti Pneumocystis carinii compound (2-[4-(4-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone) commonly known as Atovaquone and methods for producing the same is disclosed herein. This also provides pharmaceutical compositions comprising the said polymo | ||
| 7446200 | Rapid resolution process of clopidogrel base and a process for preparation of clopidogrel bisulf | November 4, 2008 |
| The present invention discloses a rapid resolution process of racemic clopidogrel base followed by conversion of the resolved (S) isomer to crystalline Clopidogrel bisulfate Form I. The invention also discloses novel racemization process of the unwanted (R) isomer of clopidogrel base | ||
| 7439365 | Pharmaceutical salt of (1-benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine (Donepezi | October 21, 2008 |
| The present invention relates to the oxalate salt of 1-benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl]methyl piperidine, commonly known as Donepezil, and its method of preparation. | ||
| 7378439 | Process for the preparation of 4-(2-dipropylaminoethyl)-1,3-dihydro-2H-indol-2-one hydrochloride | May 27, 2008 |
| The present invention discloses a novel process and novel intermediates for the Preparation of 4-[2-(di-n-propyl amino) ethyl]-1,3-dihydro-2H-indol-2-one, commonly known as Ropinirole (I) and pharmaceutical composition comprising the same. Further the present invention also discloses | ||
| 7186842 | Polymorph of (1-benzyl-4-[(5,6-dimethoxy-1-indanone)-2-y1] methyl piperidine hydrochloride (Done | March 6, 2007 |
| The present invention discloses a novel, stable polymorph of 1-benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine hydrochloride commonly known as Donepezil hydrochloride. Further the present invention discloses a process for producing Donepezil HCl amorphous and its polymor | ||
WO 2012025944
SITAGLIPTIN, SALTS AND POLYMORPHS THEREOFThe present invention relates to an improved process for preparation of Sitagliptin or pharmaceutically acceptable salts thereof. The present invention further relates to novel polymorphs of Sitagliptin salts and process for preparation thereof.
IPC: C07D487/04 applicants: AROTE NITIN DNYANESHWAR, SATHE DHANANJAY GOVIND, DAMLE SUBHASH VISHWANATH, USV LTD, AMBRE RAKESH RAMCHANDRA, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, Authors: AROTE NITIN DNYANESHWAR, DAMLE SUBHASH VISHWANATH, NAIK TUSHAR ANIL, AMBRE RAKESH RAMCHANDRA, SAWANT KAMLESH DIGAMBAR, SATHE DHANANJAY GOVIND, ...........http://www.google.co.in/patents/WO2012025944A2?cl=en


WO 2012042542
PROCESS FOR PREPARATION OF CROSSLINKED POLYMER
The present invention relates to desalination/desalting of polymer, in polyallylamine/crosslinked polymers by membrane separation techniques.IPC: C08F8/00 Applicants: SATHE DHANANJAY GOVIND, PATIL ATUL SURESH, PATIL SAMADHAN DAULAT, USV LTD, MONDKAR HARISH KASHINATH, JADHAV TANAJI SHAMRAO,
WO 2012042542
PROCESS FOR PREPARATION OF CROSSLINKED POLYMER
The present invention relates to desalination/desalting of polymer, in polyallylamine/crosslinked polymers by membrane separation techniques.IPC: C08F8/00 Applicants: SATHE DHANANJAY GOVIND, PATIL ATUL SURESH, PATIL SAMADHAN DAULAT, USV LTD, MONDKAR HARISH KASHINATH, JADHAV TANAJI SHAMRAO,
WO 2010041271
PROCESS FOR PREPARATION OF (S)-N-[2-(1,6,7,8-TETRAHYDRO-2H-INDENO[5,4-B]FURAN-8-YL)ETHYL] PROPIONAMIDE AND NOVEL INTERMEDIATES THEREOFDisclosed herein process for preparation of (S)-Ramelteon and intermediates thereof.IPC: C07D307/79 C07D307/77 Applicants: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, USV LTD, MAHAJAN ANIL DINKAR, PATNEKAR SUBODH SHASHIKANT, GATNE PARAG SUKUMAR, DEORE RAVIRAJ BHATU,
WO 2010041271
PROCESS FOR PREPARATION OF (S)-N-[2-(1,6,7,8-TETRAHYDRO-2H-INDENO[5,4-B]FURAN-8-YL)ETHYL] PROPIONAMIDE AND NOVEL INTERMEDIATES THEREOFDisclosed herein process for preparation of (S)-Ramelteon and intermediates thereof.IPC: C07D307/79 C07D307/77 Applicants: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, USV LTD, MAHAJAN ANIL DINKAR, PATNEKAR SUBODH SHASHIKANT, GATNE PARAG SUKUMAR, DEORE RAVIRAJ BHATU,
WO 2011017244
POLYMORPHS OF 5-(4-(2-(5-ETHYLPYRIDIN-2-YL)-2-OXOETHOXY)BENZYL)-1,3-THIAZOLIDINE-2,4-DIONE (MITOGLITAZONE)The present invention relates to novel polymorphs of 5-(4-(2-(5-ethylpyridin-2-yl)-2-oxoethoxy) benzyl)-l,3-thiazolidine-2,4-dione of formula (I) and processes for the preparation thereof and pharmaceutical compositions comprising the novel crystalline polymorphs. Formula (I)IPC: A61K31/44 A01N43/40 Applicants: SATHE DHANANJAY GOVIND, MANTRIPRAGADA NARAYANA RAO, METABOLIC SOLUTIONS DEV COMPAN, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, BHOPALKAR RAJESH GANPAT,
WO 2011017244
POLYMORPHS OF 5-(4-(2-(5-ETHYLPYRIDIN-2-YL)-2-OXOETHOXY)BENZYL)-1,3-THIAZOLIDINE-2,4-DIONE (MITOGLITAZONE)The present invention relates to novel polymorphs of 5-(4-(2-(5-ethylpyridin-2-yl)-2-oxoethoxy) benzyl)-l,3-thiazolidine-2,4-dione of formula (I) and processes for the preparation thereof and pharmaceutical compositions comprising the novel crystalline polymorphs. Formula (I)IPC: A61K31/44 A01N43/40 Applicants: SATHE DHANANJAY GOVIND, MANTRIPRAGADA NARAYANA RAO, METABOLIC SOLUTIONS DEV COMPAN, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, BHOPALKAR RAJESH GANPAT,
WO 2010092591
CRYSTALLINE POLYMORPHS OF 5-[[(2R,3S)-2-[(1R)-1-[3,5- BIS(TRIFLUOROMETHYL) PHENYL] ETHOXY]-3-(4-FLUOROPHENYL)-4-MORPHOLINYL]METHYL]-1,2- DIHYDRO-3H-1,2,4-TRIAZOL-3-ONE AND PROCESS FOR PREPARATION THEREOF
Disclosed herein is novel polymorph of 5-[[(2R,3S)-2-[(1R)-1-[3,5- bis(trifluoromethyl) phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-1,2- dihydro-3H-1,2,4- triazol-3-one, commonly known as Aprepitant or pharmaceutically acceptable salts thereof and process for preparation thereof. The present invention further relates to highly pure polymorph, Form II of Aprepitant. The present invention further relates to improved process for preparation of Aprepitant.
IPC: C07D413/06 Applicants: SATHE DHANANJAY GOVIND, PATIL SACHIN SHIVAJI, BHISE NANDU BABAN, USV LTD, SHINDIKAR ANAND VINOD, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, Authors: BHISE NANDU BABAN, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, SATHE DHANANJAY GOVIND, SHINDIKAR ANAND VINOD, PATIL SACHIN SHIVAJI, ............http://www.google.im/patents/WO2010092591A2?cl=enAprepitant is chemically known as 5-[[(2R,3S)-2-[(lR)-l-[3,5- bis(trifluoromethyl) phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-l,2- dihydro-3H-l ,2,4- triazol-3-one and represented by Formula IX.
WO 2010092591
CRYSTALLINE POLYMORPHS OF 5-[[(2R,3S)-2-[(1R)-1-[3,5- BIS(TRIFLUOROMETHYL) PHENYL] ETHOXY]-3-(4-FLUOROPHENYL)-4-MORPHOLINYL]METHYL]-1,2- DIHYDRO-3H-1,2,4-TRIAZOL-3-ONE AND PROCESS FOR PREPARATION THEREOF
Disclosed herein is novel polymorph of 5-[[(2R,3S)-2-[(1R)-1-[3,5- bis(trifluoromethyl) phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-1,2- dihydro-3H-1,2,4- triazol-3-one, commonly known as Aprepitant or pharmaceutically acceptable salts thereof and process for preparation thereof. The present invention further relates to highly pure polymorph, Form II of Aprepitant. The present invention further relates to improved process for preparation of Aprepitant.
IPC: C07D413/06 Applicants: SATHE DHANANJAY GOVIND, PATIL SACHIN SHIVAJI, BHISE NANDU BABAN, USV LTD, SHINDIKAR ANAND VINOD, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, Authors: BHISE NANDU BABAN, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, SATHE DHANANJAY GOVIND, SHINDIKAR ANAND VINOD, PATIL SACHIN SHIVAJI, ............http://www.google.im/patents/WO2010092591A2?cl=enAprepitant is chemically known as 5-[[(2R,3S)-2-[(lR)-l-[3,5- bis(trifluoromethyl) phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-l,2- dihydro-3H-l ,2,4- triazol-3-one and represented by Formula IX.
WO 2010041268
CROSSLINKED POLYMERS
Disclosed herein are pharmaceutical compositions comprising wet granulated bile acid sequestrants and their process of preparation. The present invention also discloses process for preparation of Colesevelam hydrochloride, an antilipemic agent.IPC: A61K31/785 A61K9/20 Applicants: SATHE DHANANJAY GOVIND, PATIL SAMADHAN DAULAT, USV LTD, OMRAY ASHOK, RAO MANTRIPRAGADA NARAYAN, MONDKAR HARISH KASHINATH, BHIDE YOGESH SHARAD, CHOUDHARY VARSHA SHASHANK, JADHAV TANAJI SHAMRAO, Authors: SATHE DHANANJAY GOVIND, PATIL SAMADHAN DAULAT, OMRAY ASHOK, RAO MANTRIPRAGADA NARAYAN, MONDKAR HARISH KASHINATH, BHIDE YOGESH SHARAD, CHOUDHARY VARSHA SHASHANK, JADHAV TANAJI SHAMRAO, ............http://www.google.com/patents/WO2010041268A2?cl=en
WO 2009118753
PROCESS FOR PREPARATION OF NARATRIPTAN HYDROCHLORIDE
The present invention relates to an improved process for the preparation of N-methyl-3- (l-methyl-4-piperidinyl)-lH-indole-5-ethanesulfonamide hydrochloride of formula (I) having less than 0.15% area by HPLC of 3-(l-methyl-4-piperidinyl)-lH-indole-5- ethanesulfonamide (IA) and intermediates thereof.IPC: C07D209/10 A61P25/06 A61P25/04 A61P43/00 A61P9/00 A61K31/404 Applicants: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, USV LTD, PATNEKAR SUBODH SHASHIKANT, DEORE RAVIRAJ BHATU, Authors: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, PATNEKAR SUBODH SHASHIKANT, DEORE RAVIRAJ BHATU,
WO 2009125433
PROCESS FOR PREPARATION OF AMINE POLYMER SALT
The present invention discloses simple process for preparation of salt of polyallylamine polymer.IPC: A61K31/785 C08F8/00 Applicants: SATHE DHANANJAY GOVIND, PATIL SAMADHAN DAULAT, USV LTD, MONDKAR HARISH KASHINATH, JADHAV TANAJI SHAMRAO, Authors: SATHE DHANANJAY GOVIND, PATIL SAMADHAN DAULAT, MONDKAR HARISH KASHINATH, JADHAV TANAJI SHAMRAO, ...http://www.google.im/patents/WO2009125433A2?cl=en
WO 2010041268
CROSSLINKED POLYMERS
Disclosed herein are pharmaceutical compositions comprising wet granulated bile acid sequestrants and their process of preparation. The present invention also discloses process for preparation of Colesevelam hydrochloride, an antilipemic agent.IPC: A61K31/785 A61K9/20 Applicants: SATHE DHANANJAY GOVIND, PATIL SAMADHAN DAULAT, USV LTD, OMRAY ASHOK, RAO MANTRIPRAGADA NARAYAN, MONDKAR HARISH KASHINATH, BHIDE YOGESH SHARAD, CHOUDHARY VARSHA SHASHANK, JADHAV TANAJI SHAMRAO, Authors: SATHE DHANANJAY GOVIND, PATIL SAMADHAN DAULAT, OMRAY ASHOK, RAO MANTRIPRAGADA NARAYAN, MONDKAR HARISH KASHINATH, BHIDE YOGESH SHARAD, CHOUDHARY VARSHA SHASHANK, JADHAV TANAJI SHAMRAO, ............http://www.google.com/patents/WO2010041268A2?cl=en
WO 2009118753
PROCESS FOR PREPARATION OF NARATRIPTAN HYDROCHLORIDE
The present invention relates to an improved process for the preparation of N-methyl-3- (l-methyl-4-piperidinyl)-lH-indole-5-ethanesulfonamide hydrochloride of formula (I) having less than 0.15% area by HPLC of 3-(l-methyl-4-piperidinyl)-lH-indole-5- ethanesulfonamide (IA) and intermediates thereof.IPC: C07D209/10 A61P25/06 A61P25/04 A61P43/00 A61P9/00 A61K31/404 Applicants: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, USV LTD, PATNEKAR SUBODH SHASHIKANT, DEORE RAVIRAJ BHATU, Authors: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, PATNEKAR SUBODH SHASHIKANT, DEORE RAVIRAJ BHATU,
WO 2009125433
PROCESS FOR PREPARATION OF AMINE POLYMER SALT
The present invention discloses simple process for preparation of salt of polyallylamine polymer.IPC: A61K31/785 C08F8/00 Applicants: SATHE DHANANJAY GOVIND, PATIL SAMADHAN DAULAT, USV LTD, MONDKAR HARISH KASHINATH, JADHAV TANAJI SHAMRAO, Authors: SATHE DHANANJAY GOVIND, PATIL SAMADHAN DAULAT, MONDKAR HARISH KASHINATH, JADHAV TANAJI SHAMRAO, ...http://www.google.im/patents/WO2009125433A2?cl=en
WO 2009063504
NOVEL CRYSTAL MODIFICATION OF EPINASTINE OR SALTS THEREOF AND PROCESS FOR PREPARATION THEREOF
The present invention provides novel crystalline forms of 3-amino-9,13b-dihydro-lH- dibenzo[c,fjimidazo[l,5-a] azepine or salts thereof and process for preparation thereof. Formula (I).IPC: C07D487/04 Applicants: SATHE DHANANJAY GOVIND, MANTRIPRAGADA NARAYANA RAO, USV LTD, SAPLE HRISHIKESH GURUNATH, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, BHOPALKAR RAJESH GANPAT, ..........http://www.google.im/patents/WO2009063504A2?cl=en
WO 2009063505
PROCESS FOR PREPARATION OF (S) (N-[[3-[3-FLUORO-4-(4-MORPHOLINYL) HEN L -2-OXO-5-OXAZOLIDIN L METHYL]ACETAMIDE
The present invention provides novel process for preparation of (S)-N-[[3-[3-Fluoro-4-(4- morpholinyl)phenyl]-2-oxo-5-oxazolidinyl] methyl] -acetamide which comprises combining (R)-N-[3-[3-fluoro-4-morpholinyl phenyl]-2-oxo-5-oxazolidinyl]methylazide in suitable solvent, acetylating agent and acid in presence of a catalyst.IPC: C07D263/20 Applicants: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, USV LTD, BHATTACHARYYA ANINDYA SIBNATH, NAIDU AVINASH VANKATRAMAN, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, ........http://www.google.im/patents/WO2009063505A2?cl=en
WO 2009063504
NOVEL CRYSTAL MODIFICATION OF EPINASTINE OR SALTS THEREOF AND PROCESS FOR PREPARATION THEREOF
The present invention provides novel crystalline forms of 3-amino-9,13b-dihydro-lH- dibenzo[c,fjimidazo[l,5-a] azepine or salts thereof and process for preparation thereof. Formula (I).IPC: C07D487/04 Applicants: SATHE DHANANJAY GOVIND, MANTRIPRAGADA NARAYANA RAO, USV LTD, SAPLE HRISHIKESH GURUNATH, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, BHOPALKAR RAJESH GANPAT, ..........http://www.google.im/patents/WO2009063504A2?cl=en
WO 2009063505
PROCESS FOR PREPARATION OF (S) (N-[[3-[3-FLUORO-4-(4-MORPHOLINYL) HEN L -2-OXO-5-OXAZOLIDIN L METHYL]ACETAMIDE
The present invention provides novel process for preparation of (S)-N-[[3-[3-Fluoro-4-(4- morpholinyl)phenyl]-2-oxo-5-oxazolidinyl] methyl] -acetamide which comprises combining (R)-N-[3-[3-fluoro-4-morpholinyl phenyl]-2-oxo-5-oxazolidinyl]methylazide in suitable solvent, acetylating agent and acid in presence of a catalyst.IPC: C07D263/20 Applicants: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, USV LTD, BHATTACHARYYA ANINDYA SIBNATH, NAIDU AVINASH VANKATRAMAN, NAIK TUSHAR ANIL, SAWANT KAMLESH DIGAMBAR, ........http://www.google.im/patents/WO2009063505A2?cl=en
Linezolid
WO 2009113092
PROCESS FOR PREPARATION OF PROGUANIL HYDROCHLORIDE
Disclosed herein is the process for the preparation of 1-(4-chlorophenyl)-5- isopropyl-biguanide hydrochloride (Proguanil hydrochloride), Formula (I), an antimalarial agent.
IPC: C07C279/26 Applicants: SATHE DHANANJAY GOVIND, USV LTD, HAGAVANE NITIN NIVRUTTI, MONDKAR HARISH KASHINATH, JADHAV TANAJI SHAMRAO, ........http://www.google.com/patents/WO2009113092A2?cl=en
Linezolid
WO 2009113092
PROCESS FOR PREPARATION OF PROGUANIL HYDROCHLORIDE
Disclosed herein is the process for the preparation of 1-(4-chlorophenyl)-5- isopropyl-biguanide hydrochloride (Proguanil hydrochloride), Formula (I), an antimalarial agent.
IPC: C07C279/26 Applicants: SATHE DHANANJAY GOVIND, USV LTD, HAGAVANE NITIN NIVRUTTI, MONDKAR HARISH KASHINATH, JADHAV TANAJI SHAMRAO, ........http://www.google.com/patents/WO2009113092A2?cl=en

WO 2008062437
PROCESS FOR THE PREPARATION OF SEVELAMER HYDROCHLORIDE AND FORMULATION THEREOF
Disclosed herein is an improved process for preparation of Sevelamer hydrochloride having phosphate binding capacity of 4.7 to 6.4mmol/g. Further, the invention discloses Sevelamer hydrochloride compositions and a novel process for preparation of said compositions comprising high shear non-aqueous granulation.IPC: A61K47/00 C08F8/44 A61K31/785 A61K9/20 A61P13/12 C08F8/00 C08F26/02 A61K9/28 Applicants: THOOVARA SASIKUMAR MOHAN, SATHE DHANANJAY GOVIND, TARUR RADHAKRISHNAN VENKATASUB, PATIL SAMADHAN DAULAT, USV LTD, HEDGE DEEPAK ANANT, MONDKAR HARISH KASHINATH, BHIDE YOGESH SHARAD, CHOUDHARY VARSHA SHASHANK, .........http://www.google.com/patents/WO2008062437A2?cl=en
WO 2008062437
PROCESS FOR THE PREPARATION OF SEVELAMER HYDROCHLORIDE AND FORMULATION THEREOF
Disclosed herein is an improved process for preparation of Sevelamer hydrochloride having phosphate binding capacity of 4.7 to 6.4mmol/g. Further, the invention discloses Sevelamer hydrochloride compositions and a novel process for preparation of said compositions comprising high shear non-aqueous granulation.IPC: A61K47/00 C08F8/44 A61K31/785 A61K9/20 A61P13/12 C08F8/00 C08F26/02 A61K9/28 Applicants: THOOVARA SASIKUMAR MOHAN, SATHE DHANANJAY GOVIND, TARUR RADHAKRISHNAN VENKATASUB, PATIL SAMADHAN DAULAT, USV LTD, HEDGE DEEPAK ANANT, MONDKAR HARISH KASHINATH, BHIDE YOGESH SHARAD, CHOUDHARY VARSHA SHASHANK, .........http://www.google.com/patents/WO2008062437A2?cl=en
WO 2009063486
PROCESS FOR PREPARATION OF DEXTROROTATORY ISOMER OF 6-(5-CHLORO-PYRID-2-YI)-5-[(4-METHYL -1-PIPERAZINYL) CARBONYLOXY]-7-OXO-6,7-DIHYDRO-5H-PYRROLO [3,4-B] PYRAZINE (ESZOPICLONE)Disclosed herein is the process for preparation of 6-(5-chloro-pyrid-2-yl)-5-[(4-methyl-1-piperazinyl) carbonyloxy]-7-oxo-6,7-dihydro-5H-pyrrolo [3,4-b] pyrazine (Zopiclone), its resolution to get the dextrorotatory isomer of formula (I) substantially free of R (-) enantiomer and recovery of key raw material i.e. 6-(5-chloro pyrid-2-yl)-5-hydroxy-7-oxo-5,6-dihydropyrrolo [3,4-b] pyrazine from the R-isomer of Zopiclone followed by conversion of the recovered compound to get pure Eszopiclone (I) in high yield and high purity.IPC: C07D487/04 Applicants: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, USV LTD, DESHPANDE MANOJ MADHUKARRAOV, SHINDIKAR ANAND VINOD, MONDKAR HARISH KASHINATH, ..........http://www.google.com/patents/WO2009063486A2?cl=en
WO 2009063486
PROCESS FOR PREPARATION OF DEXTROROTATORY ISOMER OF 6-(5-CHLORO-PYRID-2-YI)-5-[(4-METHYL -1-PIPERAZINYL) CARBONYLOXY]-7-OXO-6,7-DIHYDRO-5H-PYRROLO [3,4-B] PYRAZINE (ESZOPICLONE)Disclosed herein is the process for preparation of 6-(5-chloro-pyrid-2-yl)-5-[(4-methyl-1-piperazinyl) carbonyloxy]-7-oxo-6,7-dihydro-5H-pyrrolo [3,4-b] pyrazine (Zopiclone), its resolution to get the dextrorotatory isomer of formula (I) substantially free of R (-) enantiomer and recovery of key raw material i.e. 6-(5-chloro pyrid-2-yl)-5-hydroxy-7-oxo-5,6-dihydropyrrolo [3,4-b] pyrazine from the R-isomer of Zopiclone followed by conversion of the recovered compound to get pure Eszopiclone (I) in high yield and high purity.IPC: C07D487/04 Applicants: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, USV LTD, DESHPANDE MANOJ MADHUKARRAOV, SHINDIKAR ANAND VINOD, MONDKAR HARISH KASHINATH, ..........http://www.google.com/patents/WO2009063486A2?cl=en
WO 2008035364
PROCESS FOR THE PREPARATION OF MICRONIZED VALSARTANThe present invention relates to process for preparing micronized Valsartan with particle size distribution of d10 less than 5µ, d50 less than 10 µ and d90less than 20 µ preferably d90< 10 µ.IPC: A61K9/14 A61K31/41 Applicants: SATHE DHANANJAY GOVIND, MANTRIPRAGADA NARAYANA RAO, TARUR VENKATASUBRAMANIAN RADHAKRISHNAN, USV LTD, RANE BHUPENDRA SHALIGRAM, THOOVARA SASI KUMAR MOHAN, SAWANT KAMLESH DIGAMBAR, ..........http://www.google.im/patents/WO2008035364A2?cl=en
WO 2008093360
A PROCESS FOR PREPARATION OF (S)-(+)-N-METHYL-3(1-NAPHTHYLOXY)-3(2-THIENYL)PROPYLAMINE HYDROCHLORIDEThe present invention provides an improved process for preparation of intermediate of Duloxetine base and hydrochloride salt thereof.IPC: A61K31/381 A61P25/24 C07D333/20 Applicants: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, TARUR VENKATASUBRAMANIAN RADHAKRISHNAN, USV LTD, CHOUDHARI CHHAYENDRA JANARDAN, SRIVASTAVA NEERAJ, http://www.google.im/patents/WO2008093360A2?cl=en
WO 2008035364
PROCESS FOR THE PREPARATION OF MICRONIZED VALSARTANThe present invention relates to process for preparing micronized Valsartan with particle size distribution of d10 less than 5µ, d50 less than 10 µ and d90less than 20 µ preferably d90< 10 µ.IPC: A61K9/14 A61K31/41 Applicants: SATHE DHANANJAY GOVIND, MANTRIPRAGADA NARAYANA RAO, TARUR VENKATASUBRAMANIAN RADHAKRISHNAN, USV LTD, RANE BHUPENDRA SHALIGRAM, THOOVARA SASI KUMAR MOHAN, SAWANT KAMLESH DIGAMBAR, ..........http://www.google.im/patents/WO2008035364A2?cl=en
WO 2008093360
A PROCESS FOR PREPARATION OF (S)-(+)-N-METHYL-3(1-NAPHTHYLOXY)-3(2-THIENYL)PROPYLAMINE HYDROCHLORIDEThe present invention provides an improved process for preparation of intermediate of Duloxetine base and hydrochloride salt thereof.IPC: A61K31/381 A61P25/24 C07D333/20 Applicants: SATHE DHANANJAY GOVIND, BHISE NANDU BABAN, TARUR VENKATASUBRAMANIAN RADHAKRISHNAN, USV LTD, CHOUDHARI CHHAYENDRA JANARDAN, SRIVASTAVA NEERAJ, http://www.google.im/patents/WO2008093360A2?cl=en

Polymorphic forms of dolasetron mesylate and processes thereof
WO 2007072506 A2..........https://www.google.im/patents/WO2007072506A2
A process for the preparation of 1-(9h-carbazol-4-yloxy)-3-2-(-methoxyphenoxy)-ethyl amino-propan-2-ol
WO 2005115981 A2......https://www.google.im/patents/WO2005115981A2

Novel polymorphs of atovaquone and process of preparation thereof
WO 2006008752 A1
https://www.google.im/patents/WO2006008752A1
A process for the preparation of substantially pure 4-amino-1-isobutyl-1h-imidazo[4,5-c]-quinoline (imiquimod)
WO 2006070408 A2.......https://www.google.im/patents/WO2006070408A2
Process for preparation of rasagiline and salts thereof
EP 2364967 A2.........https://www.google.im/patents/EP2364967A2

Conferences
WO 2007072506 A2..........https://www.google.im/patents/WO2007072506A2
WO 2005115981 A2......https://www.google.im/patents/WO2005115981A2

WO 2006008752 A1
WO 2006070408 A2.......https://www.google.im/patents/WO2006070408A2
EP 2364967 A2.........https://www.google.im/patents/EP2364967A2
Conferences
The 3rd International Conference and Exhibition
Developing Chemical Processes for APIs
10 & 11 January 2011
The Residence Hotel and Convention Centre, Mumbai, India
by
Dr Dhananjay Sathe, USV Ltd, INDIA
Challenges in API Process Development for the Generic Markets: Ropinirole Experience

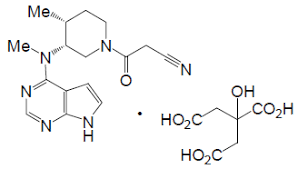
Tofacitinib Citrate
An Improved and Efficient Process for the Preparation of Tofacitinib Citrate
Yogesh S. Patil, Nilesh L. Bonde, Ankush S. Kekan, Dhananjay G. Sathe*1, and Arijit Das
Publication Date (Web): November 17, 2014 (Article)
DOI: 10.1021/op500274j
MS m/z 313 (M+ + 1);
mp 201–202 °C;
1H NMR (CDCl3) δ 8.34 (s, 1H), δ 7.38 (d, 1H, J = 2.4 Hz), δ 6.93 (d, 1H, J = 2.4 Hz), δ 4.97 (m, 1H), δ 3.93–4.03 (m, 4H), δ 3.66 (m, 1H), δ 3.50 (m, 4H), δ 2.91 (d, 2H, J = 15.6 Hz), δ 2.80 (t, 2H, J = 12.8 Hz), δ 2.55 (m, 1H), δ 1.99 (m, 1H), δ 1.77 (m, 1H), δ 1.13–1.18 (m, 3H).


Alto Porvorim, India

Alto Porvorim, India
Current city, goa india



UNICHEM
UNICHEM......http://www.unichemlabs.com/researchcentre-goa.html
Unichem Laboratories Ltd.
Plot No. 17 & 18,
Pilerne Industrial Estate,
Pilerne Bardez, Goa - 403 511
Tel: + 91 832 2407202-6
Plot No. 17 & 18,
Pilerne Industrial Estate,
Pilerne Bardez, Goa - 403 511
Tel: + 91 832 2407202-6
GOA INDIA
Goa - Wikipedia, the free encyclopedia
en.wikipedia.org/wiki/Goa
Goa is visited by large numbers of international and domestic tourists each year for its beaches, places of worship and world heritage architecture. It also has ...
RAVE
SEASGOA MINE






No comments:
Post a Comment