| 68. Schmidt, B.; Neitemeier V.; 6-Pyridylnicotine – A New Chiral 2,2’-Bipyridine. Synthesis, 1998, 42. (IF 2.2) |
| 69. Schmidt, B.; Ehlert, D. K.; Preparation of N-Boc-(2,6-bis(ethoxycarbonylpyridiny-4-yl)-L-alanines as tridentate ligands. Tet. Lett. 1998, 39, 3999-4002 |
| 70. G. Muller, B. Schmidt, J. Jiricek, G. Hopfgartner, J.P. Riehl, J.C.G. Buenzli, C. Piguet. Lanthanide triple helical complexes with a chiral ligand derived from 2,6-pyridindicarboxylic acid. J.Chem. Soc., Dalton Trans. 2001, 2658-2665 |
| 71. G. Muller, B. Schmidt; J. Jiricek, J.C.G. Buenzli, K.J. Schenk. 3-[2,6-Bis(diethylcarbamoyl)-pyridin-4-yl]-N-(tert-butoxycarbonyl)alanine methyl ester: a chiral tridentate ligand that causes a diastereomeric excess of its lanthanide complexes in solution. Acta Crystallographica, Section C: Crystal Structure Communications, 2003, 59, 353-356 |
| 72. B. Schmidt, A. Titz, J. Jiricek, Guofeng Ye, K. Parang, Copper dipicolinates as peptidomimetic ligands for the Src SH2 domain. Bioorg. Med. Chem. Lett. 2004, 14 (16), 4203-4206 |
| 73. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 6-Nitroquinolin-2(1H)-one. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(9), 2992-2993 |
| 74. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 4-Fluoro-N-(quinolin-8-yl)benzenesulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(12), o4387-o4388 |
| 75. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., N-Benzyl-8-nitroquinolin-2-amine. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(11), o3837-o3838 |
| 76. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., N-(5,7-Dibromo-8-quinolyl)-4-fluorobenzenesulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(11), o3782-o3783 |
| 77. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2,4,6-Triisopropyl-N-(8-quinolyl)benzenesulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(11), o3780-o3781 |
| 78. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 4-Nitro-N-(8-quinolyl)benzenesulfonamide.Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(11), o3778-o3779 |
| 79. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 5,7-Dibromo-N-tosylquinolin-8-amine. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(10), o3435-o3436 |
| 80. Everson da Silva, L. E.; Joussef, A. C.; Foro, S.; Schmidt, B., N-(6-Methoxy-2-methyl-8-quinolyl)-4-n-propylbenzenesulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(2), o626-o627 |
| 81. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2,2-Dimethyl-5-[(6-methylpyridin-2-yl-amino)-methylene]-1,3-dioxane-4,6-dione. Acta Crystallographica, Section E: Structure Reports Online,2006, E62(8), o3477-o3478 |
| 82. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2,2-Dimethyl-5-[(4-p-tolylthiazol-2-ylamino)-methylene]-1,3-dioxane-4,6-dione. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(8), o3215-o3216 |
| 83. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[N-(5,7-dibromoquinolin-8-yl)-3,5-bis(trifluoro-methyl)benzenesulfonamidato-k2N,N']zinc(II). Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(8), m1901-m1903 |
| 84. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Aquabis[4-nitro-N-(quinolin-8-yl)benzenesulfonamidato-k2N,N']zinc(II). Acta Crystallographica, Section E: Structure Reports Online,2006, E62(8), m1773-m1775 |
| 85. Everson da Silva, L.; Joussef, A. C.; Silva, L. L.; Foro, S.; Schmidt, B., 4-n-Propyl-N-(8-quinolyl)-benzene-sulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(9), o3606-o3607 |
| 86. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., N-(5,7-Dibromo-8-quinolyl)-3,5-difluoro-benzenesulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(7), o2630-o2631 |
| 87. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[4-fluoro-N-(quinolin-8-yl)benzenesulfonamidato-k2N,N']zinc(II) hemihydrate. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(7), m1719-m1721 |
| 88. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Aquabis[N-(5,7-dibromoquinolin-8-yl)-4-methylbenzenesulfonamidato-k2N,N']zinc(II). Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(6), m1258-m1259 |
| 89. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 5-{[4-(4-Bromophenyl)thiazol-2-yl]amino-methylene}-2,2-dimethyl-1,3-dioxane-4,6-dione. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(5), o1722-o1723 |
| 90. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[4-n-propyl-N-(8-quinolyl)benzenesulfonamidato-k2N,N']zinc(II) dimethylformamide solvate. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(5), m999-m1001 |
| 91. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[2,4,6-triisopropyl-N-(quinolin-8-yl)-benzene-sulfonamidato-k2N,N']copper(II). Acta Crystallographica, Section E: Structure Reports Online,2006, E62(4), m912-m913. |
| 92. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2-(1,3-Benzothiazol-2-yl)quinolin-8-ol. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62, (3), o880-o881 |
| 93. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[4-nitro-N-(quinolin-8-yl)benzenesulfonamidato-k2N,N'']copper(II). Acta Crystallographica, Section E: Structure Reports Online,2006, E62(3), m518-m519. |
| 94. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[2,4,6-triisopropyl-N-(quinolin-8-yl)-benzenesulfonamidato-k2N,N']zinc(II). Acta Crystallographica, Section E: Structure Reports Online,2006, E62(3), m516-m517 |
| 95. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 5-[(6-Fluoro-1,3-benzothiazol-2-ylamino)-methylene]-2,2-dimethyl-1,3-dioxane-4,6-dione. Acta Crystallographica, Section E: Structure Reports,2006, E62 (2), o742-o743. |
| 96. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 4,5-Dibromo-N-(8-quinolyl)thiophene-2-sulfonamide. Acta Crystallographica, Section E: Structure Reports Online,2006, E62(1), o309-o310 |
| 97. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2,2-Dimethyl-5-[(6-methylpyridin-2-yl-amino)-methylene]-1,3-dioxane-4,6-dione. Acta Crystallographica, Section E: Structure Reports Online,2006, E62(8), o3477-o3478 |
| 98. E. Ramic, R.-A. Eichel, K.-P. Dinse, A. Titz, B. Schmidt, Complexation of copper(II)-chelidamate – A multi-frequency pulsed Electron Paramagnetic Resonance and Electron Nuclear Double Resonance analysis J. Phys.Chem. 2006, 110(41), 20655-20663 |
| 99. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[4-fluoro-N-(quinolin-8-yl)benzenesulfonamidato-k2N,N']copper(II) dichloromethane hemisolvate. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(7), m1606-m1608 |
| 100. Everson da Silva, L.; Joussef, A. C.; Rebelo, R.A.; Foro, S.; Schmidt, B., 2-Aminoquinolin-4-yl 2,4,6-triisopropylbenzenesulfonate. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(7), o5421–o5422. |
| 101. Everson da Silva, L.; Joussef, A. C.; Rebelo, R.A.; Foro, S.; Schmidt, B., 2-Aminoquinolin-8-yl p-toluenesulfonate. Acta Crystallographica, Section E: Structure Reports Online, 2007, E63, o72–o74 |
| 102. Everson da Silva, L.; Joussef, A. C.; Rebelo, R.A.; Foro, S.; Schmidt, B., Aquabis(2-methylquinolin-8-olato-k2N,O)-zinc(II). Acta Crystallographica, Section E: Structure Reports Online, 2007, E63, m129–m132 |
| 103. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Silva, 2-Aminoquinolin-8-yl-4-fluorobenzenesulfonate. Acta Crystallographica, Section E: Structure Reports Online, 2007, E63(1), o407-o408 |
| 104. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2-Aminoquinolin-8-yl-2,4,6-triisopropylbenzenesulfonate. Acta Crystallographica, Section E: Structure Reports Online, 2007, E63(1), o409-o411 |
| 105. Everson da Silva, L.; Foro, S.; Schmidt, B., 2-Aminoquinolin-8-yl 3,5-difluorobenzenesulfonate. Acta Crystallographica, Section E: Structure Reports Online, 2007, 63(2), o829-o830 |


 Cas 1820758-44-8
C24 H18 F N3 O4 S
4′-((5-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-1,3,4-oxadiazol-2-yl-thio)-methyl)-4-fluorobiphenyl-2-carboxamide
Cas 1820758-44-8
C24 H18 F N3 O4 S
4′-((5-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-1,3,4-oxadiazol-2-yl-thio)-methyl)-4-fluorobiphenyl-2-carboxamide
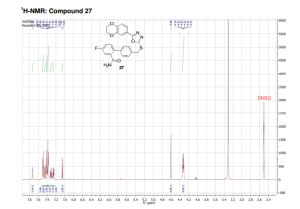
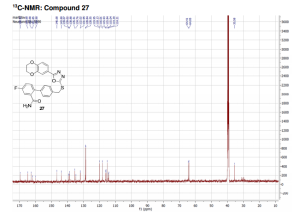 Glycogen synthase kinase-3 (GSK-3) is a constitutively active, ubiquitous serine/threonine kinase that takes part in a number of physiological processes ranging from glycogen metabolism to apoptosis. GSK-3 is a key mediator of various signaling pathways, such as the Wnt and the insulin/AKT signaling pathways.
Therefore, dysregulation of GSK-3 has been linked to various human diseases, such as cancer, diabetes, and neurodegenerative diseases.Two related isoforms of GSK-3 exist in mammals, GSK-3α and -β, which share a sequence identity within their catalytic domains of 98%.
Beyond the catalytic domains they show significant differences. Although these isoforms are structurally related, they are not functionally equivalent, and one cannot compensate for loss of the other.
The debate on the respective contributions of the isoforms GSK-3α and GSK-3β on the pathogenesis of different diseases is ongoing.
Various studies indicate that the therapies of certain diseases benefit from specific targeting of GSK-3α and GSK-3β. GSK-3α was recently identified as a differentiation target in acute myeloid leukemia (AML). AML is a hematopoietic malignancy defined by uncontrolled proliferation and disrupted myeloid differentiation. AML is the second most common form of leukemia in adults.
The current treatment of AML with conventional chemotherapy is very aggressive yet ineffective for the majority of patients with the disease.Thus, alternative targeted treatment approaches for AML are highly desirable. GSK-3α recently emerged as a potential target in this disease.
Glycogen synthase kinase-3 (GSK-3) is a constitutively active, ubiquitous serine/threonine kinase that takes part in a number of physiological processes ranging from glycogen metabolism to apoptosis. GSK-3 is a key mediator of various signaling pathways, such as the Wnt and the insulin/AKT signaling pathways.
Therefore, dysregulation of GSK-3 has been linked to various human diseases, such as cancer, diabetes, and neurodegenerative diseases.Two related isoforms of GSK-3 exist in mammals, GSK-3α and -β, which share a sequence identity within their catalytic domains of 98%.
Beyond the catalytic domains they show significant differences. Although these isoforms are structurally related, they are not functionally equivalent, and one cannot compensate for loss of the other.
The debate on the respective contributions of the isoforms GSK-3α and GSK-3β on the pathogenesis of different diseases is ongoing.
Various studies indicate that the therapies of certain diseases benefit from specific targeting of GSK-3α and GSK-3β. GSK-3α was recently identified as a differentiation target in acute myeloid leukemia (AML). AML is a hematopoietic malignancy defined by uncontrolled proliferation and disrupted myeloid differentiation. AML is the second most common form of leukemia in adults.
The current treatment of AML with conventional chemotherapy is very aggressive yet ineffective for the majority of patients with the disease.Thus, alternative targeted treatment approaches for AML are highly desirable. GSK-3α recently emerged as a potential target in this disease.





















