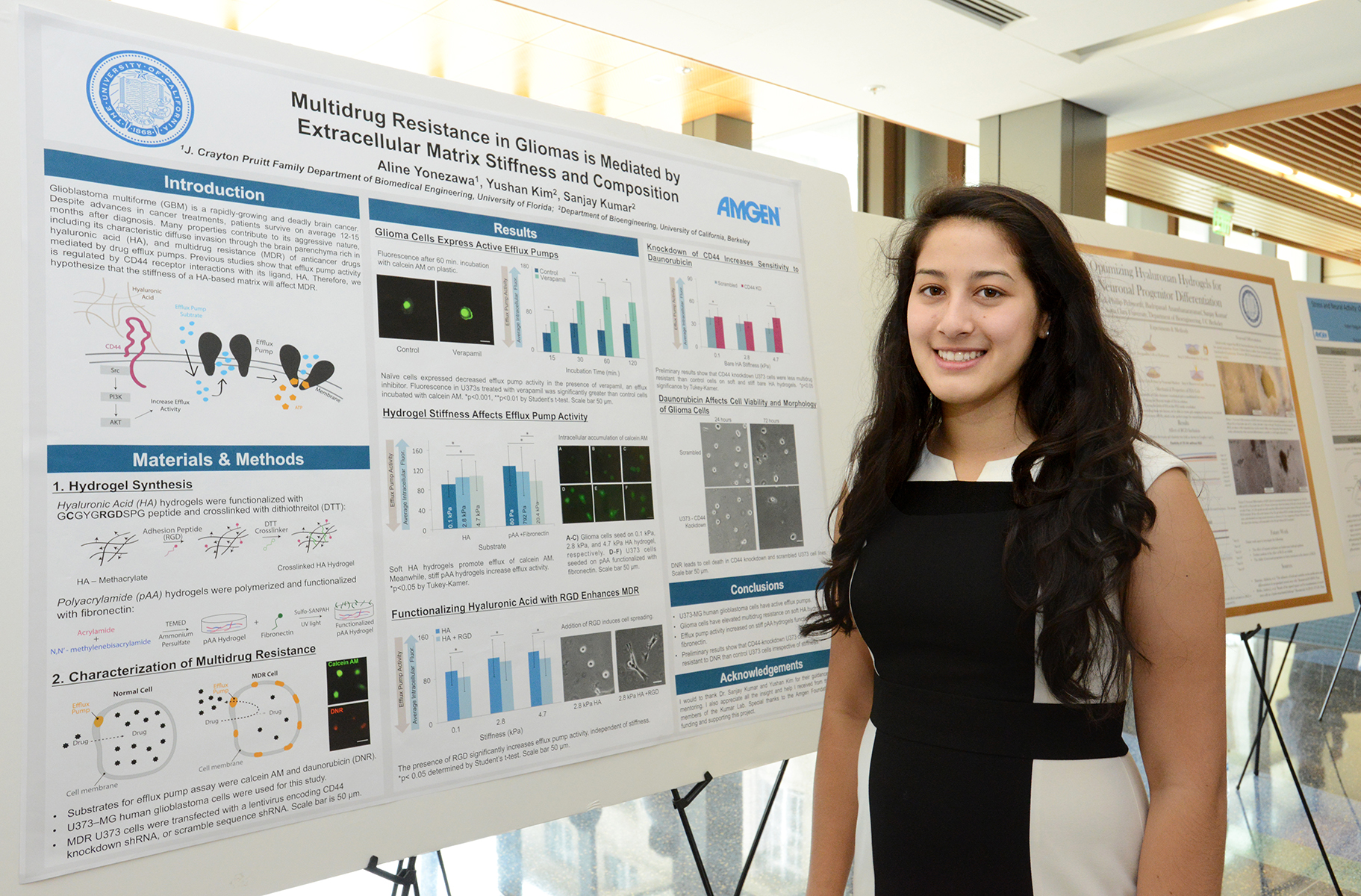
Emily Peterson, Ph.D., Amgen scientist and green chemistry team lead, applies green chemistry principles in her lab at Amgen's Cambridge Research facility.

read at http://www.genengnews.com/gen-articles/green-chemistry-initiatives-take-shape/3906/
links http://www.researchgate.net/profile/Emily_Peterson http://www.pubfacts.com/author/Emily+Peterson
https://www.linkedin.com/pub/emily-peterson/4/904/629
Emily A Peterson |
Ph.D.
|
-
Department of Medicinal Chemistry

http://www.pubfacts.com/author/Emily+Peterson
Summary
Medicinal Chemist with experience working in Oncology and Neuroscience.
Leader of Green Chemistry team at the Amgen, Cambridge facility. Our effort is currently focused on incorporating the principles of Green Chemistry into drug discovery research.
Specialties:Structure-based drug design, alkaloid synthesis

Education
Experience
Senior Scientist
Amgen
Involved in lead optimization efforts (compound design and synthesis) on an ion channel project, overseeing external chemistry resources for the project. Chemistry lead for early-stage neuroscience project. Manager of one M.S. chemist and two Ph.D. contract workers at Amgen. Green Chemistry team leader for Medicinal Chemistry at Amgen, achieved reduction of dichloromethane usage at the site by over 60%. I represent Amgen Medicinal Chemistry on the ACS Green Chemistry Institute Pharmaceutical Round Table.
Scientist
Amgen
Responsible for the design and synthesis of compounds for oncology and neuroscience projects in both the lead optimization and lead identification stages. Managed 1 B.S./M.S. chemist and a team of CRO chemists. Initiated and led the Drug Discovery Green Chemistry Team at the Amgen Massachusetts site. Involved in the interviewing and hiring of both Ph. D. and B.S./M.S. scientists.
Postdoctoral Fellow
Harvard University - Jacobsen Group
Developed a method for the asymmetric addition of indoles to cyclic N-acyl iminium ions
Patents
Preparation of dihydrobenzoxazine and tetrahydroquinoxaline sodium channel inhibitors
Europe WO 2013122897 A1 20130822
Filed August 22, 2013Preparation of bicyclic aryl and heteroaryl sodium channel inhibitors
Europe WO 2013086229 A1
Filed June 13, 2013Preparation of heteroaryl compounds as PIKK inhibitors for the treatment of cancer
Europe WO 2010132598
Issued November 18, 2010- zimidazole compounds as mTOR kinase inhibitors for the treatment of cancer
Europe WO 2010096314
Issued August 26, 2010Fused heterocyclic derivatives as HGF modulators and their preparation and methods of use
Europe WO 2009091374
Issued July 23, 2009Benzoimidazole derivatives as PI3 kinase inhibitors and their preparation, pharmaceutical compositions and use in the treatment of cancers
United States US 20090163489
Issued June 25, 2009Publications
Sustainable Chromatography (an oxymoron?)(Link)
Green Chemistry
July 15, 2014
Chromatography is routinely used in drug discovery as a means to isolate intermediates and final compounds. From a sustainability perspective, it is one of the largest contributors of solvent waste in the drug discovery process. The medicinal chemistry subgroup within the American Chemical Society's Green Chemistry Institute Pharmaceutical Roundtable (ACS GCI PR) offers a perspective aimed at...more
Sustainable Practices in Medicinal Chemistry: Current State and Future Directions.(Link)
Journal of Medicinal Chemistry
April 2013
The medicinal chemistry subgroup of the American Chemical Society’s Green Chemistry Institute Pharmaceutical Roundtable (ACS GCI PR) offers a perspective on the current state of environmentally sustainable practices in medicinal chemistry with the aim of sharing best practices more widely and highlighting some potential future developments.
Discovery and optimization of potent and selective imidazopyridine and imidazopyridazine mTOR inhibitors.(Link)
Bioorganic & medicinal chemistry letters
August 1, 2012
mTOR is a critical regulator of cellular signaling downstream of multiple growth factors. The mTOR/PI3K/AKT pathway is frequently mutated in human cancers and is thus an important oncology target. Herein we report the evolution of our program to discover ATP-competitive mTOR inhibitors that demonstrate improved pharmacokinetic properties and selectivity compared to our previous leads. Through...more
A convenient guide to help select replacement solvent for dichloromethane in chromatography(Link)
Green Chemistry
August 20, 2012
One of the largest contributors to chlorinated solvent waste in medicinal chemistry is chromatography. A set of “drug-like” compounds was employed to compare the relative eluting strengths of greener solvent systems. Disclosed herein is an experimentally-derived solvent selection guide to aid chemists in choosing greener solvents for chromatographic purification, with a particular focus on...more
Discovery of triazine-benzimidazoles as selective inhibitors of mTOR.(Link)
Bioorganic Medicinal Chemistry Letters
February 12, 2011
mTOR is part of the PI3K/AKT pathway and is a central regulator of cell growth and survival. Since many cancers display mutations linked to the mTOR signaling pathway, mTOR has emerged as an important target for oncology therapy. Herein, we report the discovery of triazine benzimidazole inhibitors that inhibit mTOR kinase activity with up to 200-fold selectivity over the structurally homologous...more
Facile preparation of protected benzylic and heteroarylmethyl amines via room temperature Curtius rearrangement(Link)
Tetrahedron Letters
May 26, 2010
A step-wise, room temperature procedure for acyl azide formation and the subsequent Curtius rearrangement of phenyl and heteroaryl acetic acids is described. We have developed a protocol for room temperature Curtius rearrangement in MeOH or CHCl3 that provides an improvement over standard conditions, avoiding the use of additives or heat. This room temperature optimization of the Curtius...more
Enantioselective, thiourea-catalyzed intermolecular addition of indoles to cyclic N-acyl iminium ions.(Link)
Angewandte Chemie International Edition
July 16, 2009
N-acyl iminium ion intermediates underwent intermolecular addition by these nucleophiles under the catalysis of a chiral thiourea Schiff base derivative. A variety of functionalized indole frameworks were assembled with high enantioselectivity from simple precursors by this method
Enantioselective Pictet−Spengler-Type Cyclizations of Hydroxylactams: H-Bond Donor Catalysis by Anion Binding(Link)
Journal of the American Chemical Society
October 17, 2007
Highly enantioenriched indolizinone and quinolizinone products are obtained in the thiourea-catalyzed cyclization of tryptamine-derived hydroxylactams. Substituent and counterion effect studies point to a novel mechanism of catalysis involving rate-limiting anion abstraction and binding by the thiourea.
Synthesis of all low-energy stereoisomers of the tris(pyrrolidinoindoline) alkaloid hodgkinsine and preliminary assessment of their antinociceptive activity.(Link)
The Journal of Organic Chemistry
September 21, 2007
The previously unknown stereoisomers 3, 4, ent-1, and ent-4 of the tris(pyrrolidinoindoline) alkaloids hodgkinsine (1) and hodgkinsine B (2) were prepared by stereocontrolled total synthesis. In each synthesis, a catalyst-controlled intramolecular Heck reaction was the key step in appending a third cis-pyrrolidinoindoline ring to a hexacyclic chimonanthine precursor. Results of the preliminary...more
Synthesis of Dihydroquinolinones Angularly-Fused to Tetrahydrobenzazepinone Rings
Heterocycles
January 2006
Emily Peterson
Senior Scientist at Amgen
Contiguous stereogenic quaternary carbons: a daunting challenge in natural products synthesis.(Link)
Proceedings of the National Academy of Sciences
August 17, 2004
One element of structure that invariably increases the difficulty of a chemical synthesis is the presence in the target molecule of contiguous all-carbon quaternary stereocenters. This Perspective will examine the most useful transformations and strategies devised recently for directly assembling this structural unit.
Synthesis of aromatic bisabolene natural products via palladium-catalyzed cross-couplings of organozinc reagents.(Link)
Journal of Organic Chemistry
May 2004
Aromatic bisabolene derivatives were prepared by two methods involving cross-coupling of organozinc reagents. The first synthesis of (±)-glandulone A (10), as well as syntheses of (±)-curcuhydroquinone (8) and (±)-curcuquinone (9), were accomplished via coupling of a secondary alkyl zinc reagent (1,5-dimethyl-4-hexenylzinc halide, 18) to protected bromohydroquinones using Pd(dppf)Cl2 as catalyst....more
Enantioselective synthesis of (−)-idiospermuline(Link)
Tetrahedron
August 25, 2003
The enantioselective total synthesis of the nonacyclic polypyrrolidinoindoline (−)-idiospermuline is described. Stereocontrolled formation of the vicinal quaternary carbon centers is achieved in a single step by dialkylation of an unsymmetrical prostereogenic dienolate with a tartrate-derived chiral dielectrophile. A catalyst-controlled diastereoselective Heck cyclization is employed to form the...more
Enantioselective total synthesis of the cyclotryptamine alkaloid idiospermuline.(Link)
Angewandte Chemie International Edition
June 5, 2003
Stereogenic quaternary carbons present the primary challenge in the syntheses of trispyrrolidinoindoline alkaloids such as idiospermuline. In its synthesis the two contiguous quaternary centers are expediently addressed by combining the lithium dienolate of a differentially protected dihydroisoindigo with a tartrate-derived electrophile. The third quaternary stereocenter is introduced by a...more
An expedient total synthesis of (±)-caparratriene(Link)
Tetrahedron Letters
July 2, 1999
(±)-Caparratriene has been synthesized by two succinct routes. The first relies on two Wittig reactions and produces (±)-caparratriene and its 2Z isomer as an inseparable 2:1 mixture. The second more efficient synthesis produces only the naturally occurring 2E isomer and proceeds in 36% overall yield. The key step in this short synthesis is the Suzuki coupling of E-2-bromo-2-butene with the E-...more