
https://www.linkedin.com/pub/mark-jensen/8/a28/ab0
Mark S. Jensen, Ph.D., Research Investigator and Group Leader in the Process Research Department at SCYNEXIS. Dr. Jensen worked for Merck in the process research department before moving to SCYNEXIS, where he continues to design and develop novel and efficient routes to early and late stage drug candidates. These processes are used at SCYNEXIS for the production of API for clinical programs. Mark holds a Ph.D. from Oregon State University and was an NIH Postdoctoral Fellow at the University of California, Irvine.
Experience
Education
An Improved Protocol for the Preparation of 3-Pyridyl- and Some Arylboronic Acids
Process
Research Department, Merck Research Laboratories, P.O. Box 2000,
Rahway, New Jersey 07065 wenjie_li@merck.com; dorian_nelson@merck.com
J. Org. Chem., 2002, 67 (15), pp 5394–5397
DOI: 10.1021/jo025792p
Publication Date (Web): June 13, 2002
Copyright © 2002 American Chemical Society
Abstract

3-Pyridylboronic
acid was prepared in high yield and bulk quantity from 3-bromopyridine
via a protocol of lithium−halogen exchange and “in situ quench”. This
technique was further studied and evaluated on other aryl halides in the
preparation of arylboronic acids.
paper
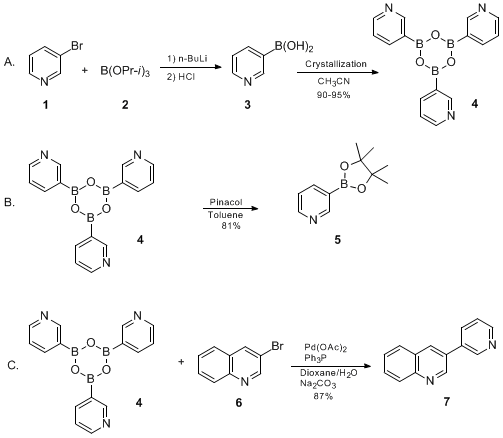 .
.compd4= The checkers obtained trispyridylboroxin in various hydration levels (0.85 - 1.0 H2O). A satisfactory melting point for this solid could not be obtained. 1H NMR pdf (400 MHz, CD3OD): δ 7.66 (br s, 1H), 8.38 (d, J=6.6, 1H), 8.51 (dd, J=1.2, 4.4, 1H), 8.61 (br s, 1 H). 1H NMR spectra were complicated in other solvents such as CDCl3 and DMSO-d6. Anal Calcd. for C15H12B3O3N3 · 1.0H2O: C, 54.15; H, 4.24; N, 12.63. Found: C, 53.95; H, 3.91; N, 12.35. Yield based on this formula is 85%. The submitters report obtaining the product in 91% yield.
compd7= The following characterization data was obtained: mp: 122-125°C; IR (KBr pellet): cm−1 3044, 1568, 1495, 1410, 1338, 1298, 1187, 1126, 1059, 1022, 952, 932, 816, 784, 758, 709, 909, 808, 786, 752, 709, 61; 1H NMR pdf (500 MHz, CDCl3): δ 7.45 (ddd, J=0.9, 4.9, 7.9, 1H), 7.60 (dt, J=0.9, 7.9, 1H), 7.75 (ddd, J=1.2, 6.8, 8.3, 1H), 7.90 (dd, J=0.9, 8.1, 1H), 8.00 (dt, J=2.2, 7.8, 1H), 8.15 (d, J=8.4, 1H), 8.32 (d, J=2.3, 1H), 8.68 (dd, J=1.5, 4.9, 1H), 8.97 (d, J=2.1, 1H), 9.15 (d, J=2.1, 1H); 13C NMR (126 MHz, CDCl3) δ 123.8, 127.3, 127.8, 128.0, 129.3, 129.9, 130.6, 133.57, 133.63, 134.6, 147.7, 148.5, 149.27, 149.29; MS (EI, 70 eV): 207 (16), 206 (M+, 100), 205 (40); HRMS (EI) m/z 206.0847, calcd for C14H10N2 206.0944. Anal. Calcd for C14H10N2: C, 81.53; H, 4.89; N, 13.58. Found: C, 81.43; H, 4.86; N, 13.40.
 5
5compd5= The checkers obtained the product in 74-82% yield in different runs. The product exhibits the following physical properties: mp 102-105°C; IR (KBr pellet) cm−1 2994, 2968, 2932, 1609, 1572, 1476, 1410, 1361, 1209, 1154, 1063, 1017, 953, 926, 859, 833, 800, 759, 705; 1H NMR pdf (500 MHz, CDCl3): δ 1.33 (s, 12H), 7.25 (ddd, J=1.1, 4.9, 7.5, 1H), 8.03 (dt, J=1.8, 7.5, 1H), 8.64 (dd, J=1.9, 4.9, 1H), 8.93 (d, J=1.1, 1H); 13C NMR (126 MHz, CDCl3) δ 24.8, 84.1, 123.0, 142.2, 152.0, 155.5; MS (EI, 70 eV): 205 (M+, 46), 204 (15), 191 (12), 190 (100), 189 (25), 162 (10), 148 (44), 147 (14), 120 (18), 119 (11), 106 (100), 105 (35), 85 (15), 59 (17), 58 (19); HRMS (EI) m/z 205.1280, calcd for C11H16NO2B 205.1274. Anal. Calcd for : C11H16BO2N: C, 64.43; H, 7.86; N, 6.83. Found: C, 64.23; H, 7.99; N, 6.88.
/////////




No comments:
Post a Comment