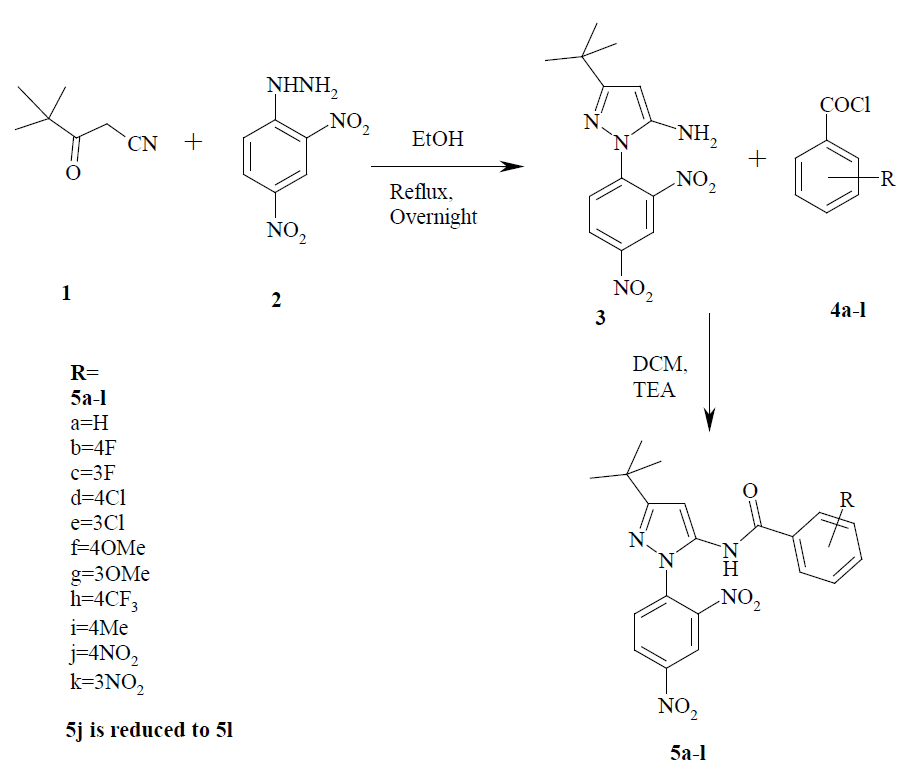
A
mixture of 4, 4-dimethyl-3-oxo-pentanenitrile (1, 34 mM), 2,
4-dinitrophenyl hydrazine hydrochloride (2, 35 mM) and 50 mL absolute
ethanol along with few drops of AcOH were heated at the reflux
temperature for overnight and cooled to room temperature. The mixture
was evaporated under vacuum and the residue thus obtained was washed
with ether, suspended in EtOAc, and treated with 1 M NaOH solution. The
organic layer then separated, washed with brine, dried over anhydrous
magnesium sulphate and concentrated. The solid which separated was
collected, then washed with a mixture of ether and hexane to give
5-amino-3-t-butyl-1-(2', 4'-dinitro)phenyl-1H-pyrazole (3) [22].
|
N-[3'-t-butyl-1'-(2", 4"-dinitro)phenylpyrazol-5'-yl] benzamide 5a: Yield: 85%, m.p.: 190-192°C,
IR (KBr) ν cm-1: 3362 cm-1 (NH str.), 3020 cm-1 (CH str. aromatic), 2950 cm-1 (CH str. methyl), 1643 cm-1 (NH bnd)
1H NMR (CDCl3) δ (ppm): 1.23 (s, 9H, t-butyl), 6.18 (s, 1H, C4, pyrazole), 7.15-7.55 (m, 3H, C3', C4', C5' aromatic), 7.7-7.8 (m, 2H, C5, C6, aromatic), 7.9-8.1 (d, 2H, C2', C6', aromatic), 8.2 (s, 1H, C3, aromatic), 9.61 (bs, 1H, NH amide).
ESI-MS (m/z): 410, 100% [M+H]+.
| The
intermediate compound 5-amino-3-t-butyl-1-(2',
4'-dinitro)phenyl-1H-pyrazole 3 was prepared in good yields by refluxing
4, 4-dimethyl-3-oxo-pentanenitrile 1 and 2, 4-dinitrophenyl hydrazine
hydrochloride 2. The FT IR spectrum of 3 showed the presence of bands
characteristic for primary amine at 3423.42 cm-1 and 3264.21 cm-1 which
are attributed to the asymmetric and symmetric stretching respectively
and aromatic hydrogen stretching band located at 3056.75 cm-1. The CH3 groups of t-butyl vibrations were observed at 2961.33 cm-1 and 2828.42 cm-1 and peak assigned for bending vibration of NH group was observed at 1625.94 cm-1. The1H
NMR of 3 revealed a broad singlet at δ 3.77 ppm characteristic for
primary amine group, multiplet at delta value of 8.05, 7.35 and 7.63 for
C3, C5 and C6 aromatic protons and a pyrazolyl-C4-H
as a singlet at 6.1 ppm. The nine t-butyl protons were found as singlet
at δ 1.23 ppm. The EI Mass spectrum of 3 showed molecular ion peak at
m/z 215. |
| When
5-amino-3-t-butyl-1-(2', 4'-dinitro)phenyl-1H-pyrazole 3 was stirred
with substituted benzoylchlorides 4a-k in dichloromethane and
triethylamine, pyrazolylbenzamides 5a-l were obtained in moderate to
good yields (5j was reduced with stannous chloride to form 5l). The
structures of the isolated compounds were determined by spectral
methods. |
| The FT IR spectrum of 5a revealed characteristic NH band at 3362 cm-1, the aromatic hydrogen stretching was found at 3020 cm-1 and stretching vibrations of CH3 group of t-butyl band was noticed at 2950 cm-1. A band at 1595 cm-1 was assigned for the C=C stretching. The 1H NMR spectra displayed a broad absorption peak at δ 9.61 which was due to resonance of NH proton of amide while the pyrazol-C4-H
and nine t-butyl protons appears as singlet’s at δ 6.18 and 1.23 ppm
respectively. The aromatic protons appeared as doublet at δ 7.9-8.1
which indicated the resonance of C2', C6' protons. Three aromatic protons C3', C4', C5' resonated as multiplet at δ 7.15-7.55 and another multiplet at δ 7.7-7.8 integrated for two protons C5 and C6. C3 proton was observed as singlet at δ 8.2. |
| Design, Synthesis, Antimicrobial and Anti-inflammatory Activity of N-Pyrazolyl Benzamide Derivatives |
| Aneesa Fatima1*, Ravindra Kulkarni2 and Bhagavanraju Mantipragada3 |
| 1Malla Reddy College of Pharmacy, Maisammaguda, Secunderabad, Telangana, India |
| 2SVERI College of Pharmacy, Gopalpur Ranjini Road, Gopalpur, Pandharpur, Maharashtra, India |
| 3Sri Venkateswara College of Pharmacy, HITEC City, Madhapur, Telangana, India |
| Corresponding Author : | Aneesa Fatima
Malla Reddy College of Pharmacy
Maisammaguda, Secunderabad
Telangana, India
Tel: +918125343156
E-mail: aneesafatima.16@gmail.com |
| Received: November 17, 2015; Accepted: December 07, 2015; Published: December 10, 2015 |
| Citation: Fatima
A, Kulkarni R, Mantipragada B (2015) Design, Synthesis, Antimicrobial
and Anti-inflammatory Activity of N-Pyrazolyl Benzamide Derivatives. Med
chem 5:521-527. doi:10.4172/2161-0444.1000311 |
|
http://www.omicsonline.org/open-access/design-synthesis-antimicrobial-and-antiinflammatory-activity-ofnpyrazolyl-benzamide-derivatives-2161-0444-1000311.php?aid=64851
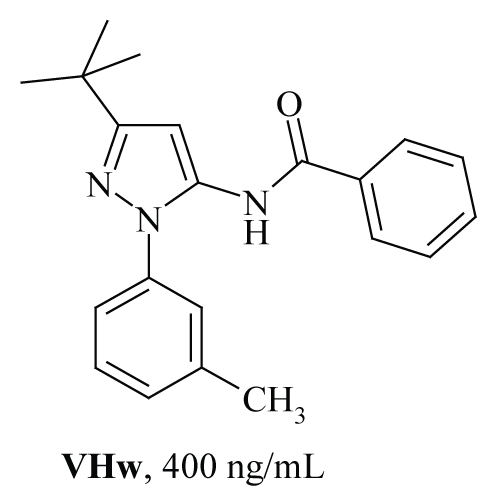 |
| Figure 1: Structure of VHw along with antibacterial activity against Bacillus subtilis (MTCC 619). |
////////////////// |


 .
.


No comments:
Post a Comment