University of Naples Federico II



.jpg) NAPOLI
NAPOLI




 ////////
////////One Organic Chemist One Day............Honouring Profiles of leading Organic Chemists brought to you by DR ANTHONY MELVIN CRASTO, worlddrugtracker, helping millions, amcrasto@gmail.com, +91 9323115463, India, skype amcrasto64



.jpg) NAPOLI
NAPOLI




 ////////
////////
 .
.

 |  |  |  |  |  |
 |  |



 Cas 1820758-44-8
C24 H18 F N3 O4 S
4′-((5-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-1,3,4-oxadiazol-2-yl-thio)-methyl)-4-fluorobiphenyl-2-carboxamide
Cas 1820758-44-8
C24 H18 F N3 O4 S
4′-((5-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-1,3,4-oxadiazol-2-yl-thio)-methyl)-4-fluorobiphenyl-2-carboxamide
 Glycogen synthase kinase-3 (GSK-3) is a constitutively active, ubiquitous serine/threonine kinase that takes part in a number of physiological processes ranging from glycogen metabolism to apoptosis. GSK-3 is a key mediator of various signaling pathways, such as the Wnt and the insulin/AKT signaling pathways.
Therefore, dysregulation of GSK-3 has been linked to various human diseases, such as cancer, diabetes, and neurodegenerative diseases.Two related isoforms of GSK-3 exist in mammals, GSK-3α and -β, which share a sequence identity within their catalytic domains of 98%.
Beyond the catalytic domains they show significant differences. Although these isoforms are structurally related, they are not functionally equivalent, and one cannot compensate for loss of the other.
The debate on the respective contributions of the isoforms GSK-3α and GSK-3β on the pathogenesis of different diseases is ongoing.
Various studies indicate that the therapies of certain diseases benefit from specific targeting of GSK-3α and GSK-3β. GSK-3α was recently identified as a differentiation target in acute myeloid leukemia (AML). AML is a hematopoietic malignancy defined by uncontrolled proliferation and disrupted myeloid differentiation. AML is the second most common form of leukemia in adults.
The current treatment of AML with conventional chemotherapy is very aggressive yet ineffective for the majority of patients with the disease.Thus, alternative targeted treatment approaches for AML are highly desirable. GSK-3α recently emerged as a potential target in this disease.
Glycogen synthase kinase-3 (GSK-3) is a constitutively active, ubiquitous serine/threonine kinase that takes part in a number of physiological processes ranging from glycogen metabolism to apoptosis. GSK-3 is a key mediator of various signaling pathways, such as the Wnt and the insulin/AKT signaling pathways.
Therefore, dysregulation of GSK-3 has been linked to various human diseases, such as cancer, diabetes, and neurodegenerative diseases.Two related isoforms of GSK-3 exist in mammals, GSK-3α and -β, which share a sequence identity within their catalytic domains of 98%.
Beyond the catalytic domains they show significant differences. Although these isoforms are structurally related, they are not functionally equivalent, and one cannot compensate for loss of the other.
The debate on the respective contributions of the isoforms GSK-3α and GSK-3β on the pathogenesis of different diseases is ongoing.
Various studies indicate that the therapies of certain diseases benefit from specific targeting of GSK-3α and GSK-3β. GSK-3α was recently identified as a differentiation target in acute myeloid leukemia (AML). AML is a hematopoietic malignancy defined by uncontrolled proliferation and disrupted myeloid differentiation. AML is the second most common form of leukemia in adults.
The current treatment of AML with conventional chemotherapy is very aggressive yet ineffective for the majority of patients with the disease.Thus, alternative targeted treatment approaches for AML are highly desirable. GSK-3α recently emerged as a potential target in this disease.

The challenge for glycogen synthase kinase-3 (GSK-3) inhibitor design lies in achieving high selectivity for one isoform over the other. The therapy of certain diseases, such as acute myeloid leukemia (AML), may require α-isoform specific targeting. The scorpion shaped GSK-3 inhibitors developed by our group achieved the highest GSK-3α selectivity reported so far but suffered from insufficient aqueous solubility. This work presents the solubility-driven optimization of our isoform-selective inhibitors using a scorpion shaped lead. Among 15 novel compounds, compound 27 showed high activity against GSK-3α/β with the highest GSK-3α selectivity reported to date. Compound 27 was profiled for bioavailability and toxicity in a zebrafish embryo phenotype assay. Selective GSK-3α targeting in AML cell lines was achieved with compound 27, resulting in a strong differentiation phenotype and colony formation impairment, confirming the potential of GSK-3α inhibition in AML therapy
http://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.5b01200
http://pubs.acs.org/doi/suppl/10.1021/acs.jmedchem.5b01200/suppl_file/jm5b01200_si_001.pdf
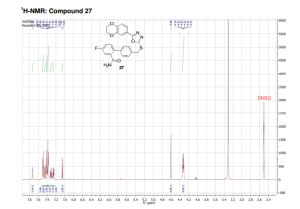
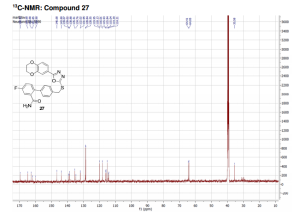
compound 27 as a colorless solid. HPLC: 96%, tR = 6.93 min.
1H NMR (DMSO-d6, 500 MHz, 300 K): δ (ppm) = 4.32 (td, J = 5.2 Hz, J = 3.7 Hz, 4H), 4.60 (s, 2H), 7.05 (d, J = 8.4 Hz, 1H), 7.25 (dd, J = 9.1 Hz, J = 2.7 Hz, 1H), 7.31 (td, J = 8.6 Hz, J = 2.8 Hz, 1H), 7.38 (m, 3H), 7.41 (d, J = 2.0 Hz, 1H), 7.45 (dd, J = 8.4 Hz, J = 2.1 Hz, 1H), 7.49 (d, J = 8.2 Hz, 2H), 7.73 (s, 1H).
13C NMR (DMSO, 125 MHz, 300 K): δ (ppm) = 35.6, 64.1, 64.4, 114.3 (d, JC–F = 21 Hz), 115.0, 115.9 (d, JC–F = 21 Hz), 115.9, 118.1, 120.0, 128.6 (2C), 128.8 (2C), 132.0 (d, JC–F = 8 Hz), 134.8, 135.5, 138.9, 139.0 (d, JC–F = 7 Hz), 143.8, 146.7, 160.9 (d, JC–F = 247 Hz), 162.7, 164.9, 169.5.
EI-MS: m/z = 463 (100, [M+]), 464 (26, [M+ + H]), 465 (7, [M+ + 2H].
ABOUT Boris Schmidt

 Cas 1820758-44-8
C24 H18 F N3 O4 S
4′-((5-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-1,3,4-oxadiazol-2-yl-thio)-methyl)-4-fluorobiphenyl-2-carboxamide
Cas 1820758-44-8
C24 H18 F N3 O4 S
4′-((5-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-1,3,4-oxadiazol-2-yl-thio)-methyl)-4-fluorobiphenyl-2-carboxamide

 Glycogen synthase kinase-3 (GSK-3) is a constitutively active, ubiquitous serine/threonine kinase that takes part in a number of physiological processes ranging from glycogen metabolism to apoptosis. GSK-3 is a key mediator of various signaling pathways, such as the Wnt and the insulin/AKT signaling pathways.
Therefore, dysregulation of GSK-3 has been linked to various human diseases, such as cancer, diabetes, and neurodegenerative diseases.Two related isoforms of GSK-3 exist in mammals, GSK-3α and -β, which share a sequence identity within their catalytic domains of 98%.
Beyond the catalytic domains they show significant differences. Although these isoforms are structurally related, they are not functionally equivalent, and one cannot compensate for loss of the other.
The debate on the respective contributions of the isoforms GSK-3α and GSK-3β on the pathogenesis of different diseases is ongoing.
Various studies indicate that the therapies of certain diseases benefit from specific targeting of GSK-3α and GSK-3β. GSK-3α was recently identified as a differentiation target in acute myeloid leukemia (AML). AML is a hematopoietic malignancy defined by uncontrolled proliferation and disrupted myeloid differentiation. AML is the second most common form of leukemia in adults.
The current treatment of AML with conventional chemotherapy is very aggressive yet ineffective for the majority of patients with the disease.Thus, alternative targeted treatment approaches for AML are highly desirable. GSK-3α recently emerged as a potential target in this disease.
Glycogen synthase kinase-3 (GSK-3) is a constitutively active, ubiquitous serine/threonine kinase that takes part in a number of physiological processes ranging from glycogen metabolism to apoptosis. GSK-3 is a key mediator of various signaling pathways, such as the Wnt and the insulin/AKT signaling pathways.
Therefore, dysregulation of GSK-3 has been linked to various human diseases, such as cancer, diabetes, and neurodegenerative diseases.Two related isoforms of GSK-3 exist in mammals, GSK-3α and -β, which share a sequence identity within their catalytic domains of 98%.
Beyond the catalytic domains they show significant differences. Although these isoforms are structurally related, they are not functionally equivalent, and one cannot compensate for loss of the other.
The debate on the respective contributions of the isoforms GSK-3α and GSK-3β on the pathogenesis of different diseases is ongoing.
Various studies indicate that the therapies of certain diseases benefit from specific targeting of GSK-3α and GSK-3β. GSK-3α was recently identified as a differentiation target in acute myeloid leukemia (AML). AML is a hematopoietic malignancy defined by uncontrolled proliferation and disrupted myeloid differentiation. AML is the second most common form of leukemia in adults.
The current treatment of AML with conventional chemotherapy is very aggressive yet ineffective for the majority of patients with the disease.Thus, alternative targeted treatment approaches for AML are highly desirable. GSK-3α recently emerged as a potential target in this disease.

| 1. Hoffmann, H. M. R.; Schmidt, B.; Wolff, S. Preparation of 5-Bromotetronates [4-Alkoxy-5-bromo-2(5H)-furanones] and a New Concept for the Synthesis of Aflatoxins and Related Structure Types. Tributyltin Hydride versus Palladium-Promoted Intramolecular Hydroarylation. Tetrahedron, 1989, 45, 6113-6126. |
| 2. Hoffmann, H. M. R.; Schmidt, B. Progress Towards a Convergent and Flexible Synthesis of AFM1. J. Toxicol.-Toxin Reviews, 1989, 8, 299-304 |
| 3. Schmidt, B.; Hoffmann, H. M. R. On the Way to Aflatoxins and Related Structure Types. Regio-controlled Annelations by Application of Homogenous Palladium Catalysis, Urethane Tether and ortho, ortho’-Diiodine Effect. Tetrahedron, 1991, 47, 9357-9368. (IF 3.40) |
| 4. Schmidt, B.; Hoffmann, H. M. R. Regioselective Preparation of Iodinated Phloroglucinols. Chem. Ber.,1992, 125, 1501-1506 |
| 5. Schmidt, B.; Chelidonic Acid as Precursor for 2,5-Desoxy-C-glycosides. Heterocycles, 1999, 51, 179-182 |
| 6. R. Raecker, M. Nicholas, B. Schmidt, O. Reiser; First determination of an activation volume for the osmium-catalyzed dihydroxylation of an alkene. J. Chem. Soc. Perk. Trans 2, 1999, 1615-1617. Räcker, Reinhard; Nicolas, Muriel; Schmidt, Boris; Reiser, Oliver. First determination of an activation volume for the osmium-catalyzed dihydroxylation of an alkene. J. Chem. Soc., Perkin Trans. 2, 1999, 2653 |
| 7. Schmidt, B.; Kühn, C. 2-Mercaptopyridine – Activated Thioates and Ketene Thioacetals. J. Prakt. Chem.1999, 341 (2), 114-120. ttp:www.wiley-vch.de/contents/jc_2258/199902.html |
| 8. H. A. Braun, R. Meusinger, B. Schmidt, 2-Iodoethanols from aldehydes, diiodomethane and isopropyl-magnesium Chloride, Tetrahedron Lett., 2005, 46 (15), 2551-2554 |
| 9. Umbreen, S.; Foro, S.; Schmidt, B., (S)-4-[2-(3-Cyanobenzamido)-3-hydroxypropyl]phenyl 3-cyano-benzoate. Acta Crystallographica, Section E: Structure Reports Online, 2006, 62(6), o2551-2552 |
| 10. Everson da Silva, L.; Joussef, A.C.; Foro, S.; Schmidt, B., 5-(Aminomethylene)-2,2-dimethyl-1,3-dioxane-4,6-dione. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(9), o3866-o3867 |
| 11. Everson da Silva, L.; Joussef, A.C.; Foro, S.; Schmidt, B., 4-n-Propyl-N-(8-quinolyl)benzenesulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(9), o3606-o3607 |
| 12. B. Schmidt, D. Meid, D. Kieser, Safe and Fast Tetrazole Formation in Ionic Liquids, Tetrahedron, 2007, 63, 492-496. http://dx.doi |
| 13. A. Zall, D. Bensinger, B. Schmidt, Oxidative homologation of aldehydes to a-ketoaldehydes by iodoform, IBX and dimethylsulfoxide. Eur. J. Org. Chem. 2012 online 24.1.2012 |
| 14. Schmidt, B.; Lindman, S.; Tong, W.; Lindeberg, G.; Gogoll, A.; Lai, Z.; Thörnwall, M.; Synnergren, B.;Nilsson, A.; Welch, C. J.; Sohtell, M.; Westerlund, C.; Nyberg, F.; Karlén, A.; Hallberg, A. Design, Synthesis and Biological Activities of Four Angiotensin II Receptor Ligands with ϒ-Turn Mimetics Replacing Amino Acid Residues 3-5. J. Med. Chem, 1997, 40, 903-919. |
| 15. Kühn, C.; Lindeberg, G.; Gogoll, A.; Hallberg, A.; Schmidt, B.; Fmoc Protected Peptide Mimetic Based on a Cyclohexane Framework and Incorporation into Angiotensin II. Tetrahedron, 1997, 53, 12497-12504 |
| 16. Schmidt, B.; Kühn, C.; Racemic, Yet Diasteromerically Pure Azido Acids as Both ϒ-Turn and Inverse ϒ-Turn Mimetics for Solid-Phase Peptide Synthesis, Synlett, 1998, 1240 |
| 17. Schmidt, B.; Patzke G. R. Fmoc-N-allyl Glycine Derived N-Allyl-2,5-diketopiperazines. Synthetic Communications, 1999, 29(6), 1025-1032 |
| 18. B. Schmidt, C. Kühn, D.K. Ehlert, G. Lindeberg, S. Lindman, A. Karlén, A. Hallberg, A Frame Shifted Disulfide Bridged Analogue of Angiotensin II. Bioorg. Med. Chem., 2003, 11, 985-990 |
| 19. Kramer, C., J. Sunkomat, B. Schmidt, B, Schieffer et al.. Angiotensin II Receptor-Independent Antiinflammatory and Antiaggregatory Properties of Losartan. Role of the Active Metabolite EXP3179.Circulation Research, 2002, 770-77 |
| 20. B. Schieffer, B. Schmidt, H. Drexler, Angiotensin-II-Rezeptor-unabhängige anti-inflammatorische und antiaggregatorische Eigenschaften von AT1-Antagonisten. Rolle aktiver Metaboliten. Herz BNK, 2003, 28 (7), 2 |
| 21. M. Schupp, L. D. Lee, N. Frost, S. Umbreen, B. Schmidt, T. Unger, U. Kintscher. CHBPR-Regulation of PPARg Activity by Losartan Metabolites. Hypertension, 2006, 47(3), 586-9 |
| 22. C. Grothusen, S. Umbreen, I. Konrad, K. Stellos, C. Schulz, B. Schmidt, E. Kremmer, O. Teebken, S. Massberg, M. Luchtefeld, B. Schieffer*, M. Gawaz. EXP3179 Inhibits Collagen-Dependent Platelet Activation via Glycoprotein Receptor-VI Independent of AT1-Receptor Antagonism. Arteriosclerosis, Thrombosis & Vascular Biology, 2007, 27(5), 1184-1190 |
| 23. L. Danielyan*, A. Lourhmati, S.Verleysdonk, B. Proksch, S.Umbreen, B. Schmidt, C.H. Gleiter. Angiotensin receptor type 1 blockade in astrocytes decreases hypoxia-induced cytotoxicity and inflammation. Neurochemical Research, 2007, 9, 1489-1498. |
| 24. D. Werner, U. Werner, A. Meybaum, B. Schmidt, S. Umbreen, A. Grosch, H. G. Lestin, B. Graf, O. Zolk; M.F. Fromm. Determinants of steady-state torasemide pharmacokinetics: Impact of gender, pharmacogenetic factors and angiotensin II receptor blockers. Clinical Pharmacokinetics, 2008, 47(5), 323-32. (IF 4.12) 20 S Proteasome |
| 25. B. Schmidt, Hannes A. Braun, E-1,2-Dichlorovinyl ethers as irreversible protease inhibitors. Tet. Lett,2004, 45 (8), 1751-1753 |
| 26. H. A. Braun, S. Umbreen, M. Groll, U. Kuckelkorn, I. Mlynarczuk, M. E. Wiegand, I. Drung, P. M. Kloetzel, B. Schmidt. Tripeptide mimetics inhibit the 20S proteasome by covalent bonding to the active site threonines J. Biol Chem, 2005, 280 (31), 28394-28411 |
| 27. I. Mlynarczuk-Bialy, H. Roeckmann, U. Kuckelkorn, B. Schmidt, S. Umbreen, J. Golab, A. Ludwig, C. Montag, L. Wiebusch, C Hagemeier, D. Schadendorf, P.-M. Kloetzel, U. Seifert. Combined Effect of Proteasome and Calpain Inhibition on Cisplatin-Resistant Human Melanoma Cells. Cancer Research, 2006, 66(15), 7598-7605 |
| 28. M.A. Graewert, N. Gallastegui, M. Stein, B. Schmidt, P.-M. Kloetzel, R. Huber, M. Groll. Elucidation of the α-Keto-Aldehyde Binding Mechanism: A Lead Structure Motif for Proteasome Inhibition. Angew. Chemie 2011, 123(2), 563-566, Angew. Chemie IE, 2011, 50(2), 542-546 |
| 29. B. Schmidt, A. Zall, G. Larbig, Inhibitors Designed for Presenilin 1 by Means of Aspartic Acid Activation. Helvetica Chimica Acta , 2004, 87, 2334-2340 |
| 30. B. Schmidt, S. Baumann, R. Narlawar, H.A. Braun, G. Larbig. Modulators and Inhibitors of ϒ- and β-Secretase. Neurodegenerative Diseases, 2006, 3(4-5), 290-297 |
| 31. G. Larbig, B. Schmidt. A Facile Synthesis of Tetramic & Tetronic Acids as β-Secretase Inhibitors, J. Combinatorial Chemistry , 2006, 8, 480-490 |
| 32. S. Umbreen, M. Brockhaus, H. Ehrenberg, B. Schmidt. Norstatines from Aldehydes by Sequential Organocatalytic α-Amination and Passerini Reaction. European J. Org. Chem. 2006, 4585-4595 |
| 33. R. Narlawar, K.-H. Baumann, R. Schubenel, B. Schmidt. Curcumin Derivatives inhibit or modulate β-amyloid precursor protein metabolism. Neurodegen. Dis. 2007, 4(2), 88-93 |
| 34. R. Narlawar, B. Perez Revuelta, K. Baumann, R. Schubenel, C. Haass, H. Steiner, B. Schmidt*. N-Substituted Carbazolyloxyacetic Acids Modulate Alzheimer Associated ϒ–Secretase. Biorg. Med. Chem. Lett. 2007, 17(1), 176-182 |
| 35. R. Narlawar, B. Perez Revuelta, C. Haass, H. Steiner, K.-H. Baumann, B. Schmidt*. The Scaffold of the COX-2 Inhibitor Carprofen Provides Alzheimer ϒ-Secretase Modulators. J. Med. Chem. 2006, 49(26), 7588-7591 |
| 36. G. Larbig, M. Pickhardt, D.G. Lloyd, B. Schmidt*, E. Mandelkow. Screening for inhibitors of tau protein aggregation into Alzheimer paired helical filaments: A ligand based approach results in successful scaffold hopping. Current Alzheimer Research 2007, 4(3), 315-323 |
| 37. V. Limongelli, L. Marinelli*, S. Cosconati, H. A. Braun, B. Schmidt, E. Novellino. Ensemble-docking approach on BACE-1: Pharmacophore Perception and Directives for Drug Development. ChemMedChem,2007, 2(5), 667-678 |
| 38. M. Pickhardt, G. Larbig, I. Khlistunova, A. Coksezen, B. Meyer, E-M. Mandelkow, B. Schmidt*, E. Mandelkow*. Phenylthiazolyl-hydrazide and its derivatives are potent inhibitors of tau aggregation and toxicity in vitro and in cells. Biochemistry, 2007, 46(35), 10016 -10023 |
| 39. R. Narlawar, K. Baumann, C. Czech, B. Schmidt. Conversion of the LXR-Agonist TO-901317 – From Inverse to Normal Modulation of g-Secretase by Addition of a Carboxylic Acid and a Lipophilic Anchor.Biorg. Med. Chem. Lett. 2007, 17(19), 5428-5431 |
| 40. H. A. Braun, A. Zall, M. Brockhaus, M. Schütz, R. Meusinger, B. Schmidt. Aspartic Protease Inhibitors via C1 -Homologation of Peptidic Aldehydes and Studies on Reduced Amide Isosteres. Tet. Lett. 2007, 48(45) 7990-7993 |
| 41. R. Narlawar, M. Pickhardt, S. Leuchtenberger, K. Baumann, S. Krause, T. Dyrks, S. Weggen, E. Mandelkow, B. Schmidt*. Curcumin Derived Pyrazoles and Isoxazoles – Swiss Army Knives or Blunt Tools for Alzheimer´s Disease? ChemMedChem, 2008, 3, 165-172 |
| 42. T. L. Kukar, T. B. Ladd, M. A. Bann, P. C. Fraering, R. Narlawar, G. M. Maharvi, B. Healy, R.Chapman, A. Welzel, R. W. Price, B. Moore, V. Rangachar, B. Cusack, J. Eriksen, K. Jansen-West, C. Verbeeck, D. Yager, C. Eckman, W. Ye, S. Sagi, B. A. Cottrell, J.Torpey, T. L. Rosenberry, A. Fauq, M. S. Wolfe, B. Schmidt, D. M. Walsh, Edward H. Koo, T.E. Golde. Substrate targeting ϒ-Secretase Modulators. Nature, 2008, 453, 7197 |
| 43. S. Baumann, N. Hoettecke R. Schubenel, K. Baumann, B. Schmidt. NSAID-derived ϒ-secretase modulators. Part III. Membrane anchoring. Biorg. Med. Chem. Lett. 2009, 19 (24), 19, 6986-6990 |
| 44. J. Pruessmeyer, C. Martin, F. M. Hess, N. Schwarz, S. Schmidt, T. Kogel, N. Hoettecke, B. Schmidt, A. Sechi, S. Uhlig, A. Ludwig. A Disintegrin and Metalloproteinase 17 (ADAM17) Mediates Inflammation-induced Shedding of Syndecan-1 and -4 by Lung Epithelial Cells. J. Biol. Chem. 2010, 285(1), 555-564 |
| 45. N. Hoettecke, A. Ludwig, S. Foro, B. Schmidt. Improved Synthesis of ADAM10 Inhibitor GI254023X.Neurodegenerative Diseases, 2010, 7(4), 232-238 |
| 46 N. Hoettecke, M. Liebeck, K. Baumann, R. Schubenel, E. Winkler, H. Steiner, B. Schmidt. Inhibition of ϒ-secretase by the CK1 inhibitor IC261 does not depend on 1d, Biorg. Med. Chem. Lett. 2010, 20(9), 2958-2963 |
| 47. S. Burgold, T. Bittner, M. M. Dorostkar, D.Kieser, M. Fuhrmann, G. Mitteregger, H. Kretzschmar, B. Schmidt, J. Herms. In vivo multiphoton imaging reveals gradual growth of newborn amyloid plaques over weeks Acta Neuropathologica, 2011, 121, 327-335 |
| 48. F. Lo Monte, T. Kramer, A. Boländer, B. Plotkin, H. Eldar-Finkelman, A. Fuertes, J. Dominguez, B. Schmidt. Synthesis and biological evaluation of glycogen synthase kinase 3 (GSK-3) inhibitors: a fast and atom efficient access to 1-aryl-3-benzylureas. Bioorg. Med. Chem. Lett. 2011, 21(18), 5610-5615 |
| 49. A. Zall, D. Kieser, N. Höttecke, E. C. Naumann, B. Thomaszewski, K. Schneider, D.T. Steinbacher, R. Schubenel, S. Masur, K. Baumann, B. Schmidt. NSAID-derived ϒ-secretase modulation requires an acidic moiety on the carbazole scaffold. Bioorg. Med. Chem. 2011, 19(16), 4903-4911 |
| 50. A. Taghavi, S. Nasir, M. Pickhardt, R. Heyny-von Haußen, G. Mall, E. Mandelkow, E.-M. Mandelkow, B. Schmidt. N'-benzylidene-benzohydrazides as novel and selective tau-PHF ligands. J. Alzheimer’s Disease,2011, 27 (4), 835-843 |
| 51. F. Lo Monte, T. Kramer, J. Gu, U. Anumala, L. Marinelli, V. La Pietra, E. Novellino, B. Franco, D. Demedts, F. Van Leuven, A. Fuertes, J. Dominguez, B. Plotkin, H. Eldar-Finkelman, B. Schmidt.Identification of Glycogen Synthase Kinase-3 Inhibitors with a Selective Sting for Glycogen Synthase Kinase-3α. J. Medicinal Chemistry, 2012, 55 (9), 4407–4424 |
| 52. A. Boländer, D. Kieser, C. Voss, S. Bauer, C. Schön, S. Burgold, T. Bittner, J. Hölzer, R. Heyny-von Haußen, G. Mall, V. Goetschy, C. Czech, H. Knust, R. Berger, J. Herms, I. Hilger, B. Schmidt. Bis(arylvinyl)pyrazines, -pyrimidines and -pyridazines as Imaging Agents for Tau Fibrils and β-Amyloid Plaques in Alzheimer’s Disease Models. J. Med. Chem. 2012, 55 (21), 9170-9180 |
| 53. T. Bittner, S. Burgold, M. M. Dorostkar, M. Fuhrmann, B. M. Wegenast-Braun, B. Schmidt, H. Kretzschmar, J. Herms. Amyloid plaque formation precedes dendritic spine loss. Acta Neuropathologica,2012, 124 (6), 797-808 |
| 54. J. Gu, U. R. Anumala, F. Lo Monte, T. Kramer; R. Heyny-von Haußen, J. Hölzer; V. Goetschy-Meyer; G. Mall, I. Hilger, C. Czech, B. Schmidt. 2-Styrylindolium based fluorescent probes visualize neurofibrillary tangles in Alzheimer's disease. Bioorg. Med. Chem. Lett. 2012, 22 (24), 7667-7671 |
| 55. C. Schön, N.A. Hoffmann, S.M. Ochs, S. Burgold, S. Filser, S. Steinbach, M.W. Seeliger, T. Arzberger, M. Goedert, H.A. Kretzschmar, B.Schmidt, J. Herms. Long-Term In Vivo Imaging of Fibrillar Tau in the Retina of P301S Transgenic Mice. PLoS ONE, 2012, 7(12): e53547. doi:10.1371/journal.pone.0053547 |
| 56. F. Lo Monte, T. Kramer, J. Gu, M. Brodrecht, J. Pilakowski, Ana Fuertes, J. M. Dominguez, B. Plotkin, H. Eldar-Finkelman, B. Schmidt, Structure-based optimization of oxadiazole-based GSK-3 inhibitors.European J. Med. Chem. 2013, 61, 26-40 |
| 57. J. Gu, U.R. Anumala, R. Heyny-von Haußen, J. Hölzer, V. Goetschy-Meyer, G. Mall, I. Hilger, C. Czech, B. Schmidt. Design, Synthesis and Biological Evaluation of Trimethine Cyanine Dyes as Fluorescent Probes for the Detection of Tau Fibrils in Alzheimer´s Disease Brain and Olfactory Epithelium. ChemMedChem,2013, 8, 891-897 |
| 58. A. Boländer, D. Kieser, C. Scholz, R. Heyny-von Haußen, G. Mall, V. Goetschy, C. Czech B. Schmidt. Synthesis of Methoxy-X04 Derivatives and their Evaluation in Alzheimer's Disease Pathology.Neurodegenerative Diseases, 2013, accepted 18.4.2013 |
| 59. E. C. Naumann, S. Göring, I. Ogorek, S. Weggen, B. Schmidt. Membrane anchoring ϒ-secretase modulators with terpene-derived moieties. Bioorg. Med. Chem. Lett. 2013, 23, 3852-3856 |
| 60. |
| 61. Luiz Everson da Silva, Antônio Carlos Joussef, Letícia Kramer Pacheco, Daniela Gaspar da Silva, Mário Steindel, Ricardo Andrade Rebelo and Boris Schmidt, Synthesis and in vitro evaluation of leishmanicidal and trypanocidal activities of N-quinolin-8-yl-arylsulfonamides. Bioorg. Med. Chem. 2007, 15, 7553–7560. Cystic fibrosis / protein folding / natural products |
| 62. Karen Bernard, Wei Wang, Rajeshwar Narlawar, Boris Schmidt, Kevin L. Kirk. Curcumin cross-links cystic fibrosis transmembrane conductance regulator (CFTR) polypeptides and potentiates CFTR channel activity by distinct mechanisms. J. Biol. Chem. 2009, 284, 30754-3076 |
| 63. Alison M. Cooper, Philip S. Hobson, Mark R. Jutton, Michael W. Kao, Binia Thomaszewski, Boris Schmidt, David J. Fear, Andrew J. Beavil, James M. McDonnell, Brian J. Sutton & Hannah J. Gould. Soluble CD23 Controls IgE Synthesis and Homeostasis in Human B Cells. J. of Immunology, 2012, 188 (7), 3199-3207 |
| 64. V. Lo Sardo, C. Zuccato, G. Gaudenzi, B. Vitali, C. Ramos, M. Tartari, M.A. Myre, J.A. Walker, A. Postocchi, L. Conti, M. Valenza, B. Drung, B. Schmidt, J. Gusella, S. Zeitlin, F. Cotelli, E. Cattaneo. Nature Neuroscience, 2012, An evolutionary recent neuroepithelial cell adhesion function of huntingtin implicates ADAM10-Ncadherin, 15 (5), 713-721. (IF 14.19) |
| 65. Q. Xiao, F. Zhang, L. Lin, C. Fang, G. Wen, T.-N. Tsai, X. Pu, D. Sims, Z. Zhang, X. Yin, B. Thomaszewski, B. Schmidt. M. Mayr, K. Suzuki, Q. Xu, S. Ye. A Functional Role of Matrix Metalloproteinase-8 in Stem/Progenitor Cell Migration and Their Recruitment into Atherosclerotic Lesions. Circulation Research,2013, 112, 35 (IF 9.9). |
| 66. Q. Xiao, F. Zhang, G. Grassia, Y. Hu, Z. Zhang, Q. Xing, X. Yin, M. Maddaluno, B. Drung, B. Schmidt, P. Maffia, A. Ialenti, M. Mayr, Q. Xu, S. Ye. Matrix Metalloproteinase-8 Promotes Vascular Smooth Muscle Cell Proliferation and Neointima Formation. Arterioscler. Thromb. Vasc. Biol. 2013, published online October 24 2013, (IF 6.338) doi:10.1161/ATVBAHA.113.301418 |
| 67. Amombo, G. M. O.; Kramer, T.; Lo Monte, F.; et al.. Modification of a promiscuous inhibitor shifts the inhibition from gamma-secretase to FLT-3 . Bioorganic & Medicinal Chemistry Letters, 2012, 22 (24), 7634-7640 |
| 68. Schmidt, B.; Neitemeier V.; 6-Pyridylnicotine – A New Chiral 2,2’-Bipyridine. Synthesis, 1998, 42. (IF 2.2) |
| 69. Schmidt, B.; Ehlert, D. K.; Preparation of N-Boc-(2,6-bis(ethoxycarbonylpyridiny-4-yl)-L-alanines as tridentate ligands. Tet. Lett. 1998, 39, 3999-4002 |
| 70. G. Muller, B. Schmidt, J. Jiricek, G. Hopfgartner, J.P. Riehl, J.C.G. Buenzli, C. Piguet. Lanthanide triple helical complexes with a chiral ligand derived from 2,6-pyridindicarboxylic acid. J.Chem. Soc., Dalton Trans. 2001, 2658-2665 |
| 71. G. Muller, B. Schmidt; J. Jiricek, J.C.G. Buenzli, K.J. Schenk. 3-[2,6-Bis(diethylcarbamoyl)-pyridin-4-yl]-N-(tert-butoxycarbonyl)alanine methyl ester: a chiral tridentate ligand that causes a diastereomeric excess of its lanthanide complexes in solution. Acta Crystallographica, Section C: Crystal Structure Communications, 2003, 59, 353-356 |
| 72. B. Schmidt, A. Titz, J. Jiricek, Guofeng Ye, K. Parang, Copper dipicolinates as peptidomimetic ligands for the Src SH2 domain. Bioorg. Med. Chem. Lett. 2004, 14 (16), 4203-4206 |
| 73. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 6-Nitroquinolin-2(1H)-one. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(9), 2992-2993 |
| 74. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 4-Fluoro-N-(quinolin-8-yl)benzenesulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(12), o4387-o4388 |
| 75. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., N-Benzyl-8-nitroquinolin-2-amine. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(11), o3837-o3838 |
| 76. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., N-(5,7-Dibromo-8-quinolyl)-4-fluorobenzenesulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(11), o3782-o3783 |
| 77. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2,4,6-Triisopropyl-N-(8-quinolyl)benzenesulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(11), o3780-o3781 |
| 78. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 4-Nitro-N-(8-quinolyl)benzenesulfonamide.Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(11), o3778-o3779 |
| 79. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 5,7-Dibromo-N-tosylquinolin-8-amine. Acta Crystallographica, Section E: Structure Reports Online, 2005, E61(10), o3435-o3436 |
| 80. Everson da Silva, L. E.; Joussef, A. C.; Foro, S.; Schmidt, B., N-(6-Methoxy-2-methyl-8-quinolyl)-4-n-propylbenzenesulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(2), o626-o627 |
| 81. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2,2-Dimethyl-5-[(6-methylpyridin-2-yl-amino)-methylene]-1,3-dioxane-4,6-dione. Acta Crystallographica, Section E: Structure Reports Online,2006, E62(8), o3477-o3478 |
| 82. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2,2-Dimethyl-5-[(4-p-tolylthiazol-2-ylamino)-methylene]-1,3-dioxane-4,6-dione. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(8), o3215-o3216 |
| 83. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[N-(5,7-dibromoquinolin-8-yl)-3,5-bis(trifluoro-methyl)benzenesulfonamidato-k2N,N']zinc(II). Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(8), m1901-m1903 |
| 84. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Aquabis[4-nitro-N-(quinolin-8-yl)benzenesulfonamidato-k2N,N']zinc(II). Acta Crystallographica, Section E: Structure Reports Online,2006, E62(8), m1773-m1775 |
| 85. Everson da Silva, L.; Joussef, A. C.; Silva, L. L.; Foro, S.; Schmidt, B., 4-n-Propyl-N-(8-quinolyl)-benzene-sulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(9), o3606-o3607 |
| 86. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., N-(5,7-Dibromo-8-quinolyl)-3,5-difluoro-benzenesulfonamide. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(7), o2630-o2631 |
| 87. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[4-fluoro-N-(quinolin-8-yl)benzenesulfonamidato-k2N,N']zinc(II) hemihydrate. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(7), m1719-m1721 |
| 88. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Aquabis[N-(5,7-dibromoquinolin-8-yl)-4-methylbenzenesulfonamidato-k2N,N']zinc(II). Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(6), m1258-m1259 |
| 89. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 5-{[4-(4-Bromophenyl)thiazol-2-yl]amino-methylene}-2,2-dimethyl-1,3-dioxane-4,6-dione. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(5), o1722-o1723 |
| 90. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[4-n-propyl-N-(8-quinolyl)benzenesulfonamidato-k2N,N']zinc(II) dimethylformamide solvate. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(5), m999-m1001 |
| 91. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[2,4,6-triisopropyl-N-(quinolin-8-yl)-benzene-sulfonamidato-k2N,N']copper(II). Acta Crystallographica, Section E: Structure Reports Online,2006, E62(4), m912-m913. |
| 92. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2-(1,3-Benzothiazol-2-yl)quinolin-8-ol. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62, (3), o880-o881 |
| 93. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[4-nitro-N-(quinolin-8-yl)benzenesulfonamidato-k2N,N'']copper(II). Acta Crystallographica, Section E: Structure Reports Online,2006, E62(3), m518-m519. |
| 94. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[2,4,6-triisopropyl-N-(quinolin-8-yl)-benzenesulfonamidato-k2N,N']zinc(II). Acta Crystallographica, Section E: Structure Reports Online,2006, E62(3), m516-m517 |
| 95. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 5-[(6-Fluoro-1,3-benzothiazol-2-ylamino)-methylene]-2,2-dimethyl-1,3-dioxane-4,6-dione. Acta Crystallographica, Section E: Structure Reports,2006, E62 (2), o742-o743. |
| 96. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 4,5-Dibromo-N-(8-quinolyl)thiophene-2-sulfonamide. Acta Crystallographica, Section E: Structure Reports Online,2006, E62(1), o309-o310 |
| 97. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2,2-Dimethyl-5-[(6-methylpyridin-2-yl-amino)-methylene]-1,3-dioxane-4,6-dione. Acta Crystallographica, Section E: Structure Reports Online,2006, E62(8), o3477-o3478 |
| 98. E. Ramic, R.-A. Eichel, K.-P. Dinse, A. Titz, B. Schmidt, Complexation of copper(II)-chelidamate – A multi-frequency pulsed Electron Paramagnetic Resonance and Electron Nuclear Double Resonance analysis J. Phys.Chem. 2006, 110(41), 20655-20663 |
| 99. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Bis[4-fluoro-N-(quinolin-8-yl)benzenesulfonamidato-k2N,N']copper(II) dichloromethane hemisolvate. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(7), m1606-m1608 |
| 100. Everson da Silva, L.; Joussef, A. C.; Rebelo, R.A.; Foro, S.; Schmidt, B., 2-Aminoquinolin-4-yl 2,4,6-triisopropylbenzenesulfonate. Acta Crystallographica, Section E: Structure Reports Online, 2006, E62(7), o5421–o5422. |
| 101. Everson da Silva, L.; Joussef, A. C.; Rebelo, R.A.; Foro, S.; Schmidt, B., 2-Aminoquinolin-8-yl p-toluenesulfonate. Acta Crystallographica, Section E: Structure Reports Online, 2007, E63, o72–o74 |
| 102. Everson da Silva, L.; Joussef, A. C.; Rebelo, R.A.; Foro, S.; Schmidt, B., Aquabis(2-methylquinolin-8-olato-k2N,O)-zinc(II). Acta Crystallographica, Section E: Structure Reports Online, 2007, E63, m129–m132 |
| 103. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., Silva, 2-Aminoquinolin-8-yl-4-fluorobenzenesulfonate. Acta Crystallographica, Section E: Structure Reports Online, 2007, E63(1), o407-o408 |
| 104. Everson da Silva, L.; Joussef, A. C.; Foro, S.; Schmidt, B., 2-Aminoquinolin-8-yl-2,4,6-triisopropylbenzenesulfonate. Acta Crystallographica, Section E: Structure Reports Online, 2007, E63(1), o409-o411 |
| 105. Everson da Silva, L.; Foro, S.; Schmidt, B., 2-Aminoquinolin-8-yl 3,5-difluorobenzenesulfonate. Acta Crystallographica, Section E: Structure Reports Online, 2007, 63(2), o829-o830 |
| 106. B. Schmidt, Methyltrioxorhenium – From Oxidation and Cyclopropanation to Metathesis. J. Prakt. Chem. 1997, 339, 493-504 |
| 107. B. Schmidt, Aspartic Proteases involved in Alzheimer's disease, ChemBioChem, 2003, 4 (5), 367-378. (Impact factor 2.9) |
| 108. B. Schmidt, B. Schieffer, Angiotensin II AT1 Receptor Antagonists: Clinical Implications of Active Metabolites. J. Med. Chem. 2003, 46, 2261. (Impact factor 5.3) |
| 109. B. Schmidt, H. Drexler, B. Schieffer, Therapeutic Effects of Angiotensin (AT1) Receptor Antagonists: Potential Contribution of Mechanisms Other Than AT1 Receptor Blockade. American Journal of Cardiovascular Drugs, 2004, 4, 361-368 |
| 110. B. Schmidt, R. Narlawar, H. Braun, Drug Development and PET-Diagnostics for Alzheimer's Disease. Current Medicinal Chemistry. 2005, 12, 1677-1695 |
| 111. B. Schmidt, S. Baumann, H.A. Braun, G. Larbig, Inhibitors and Modulators of β- and ϒ-Secretase.Current Topics in Medicinal Chemistry. 2006, 6(4), 377-392 |
| 112. B. Bulic, M. Pickhardt, B. Schmidt, E.M. Mandelkow, H. Waldmann, E. Mandelkow. Development of Tau Aggregation Inhibitors for Alzheimer's Disease. Angew. Chem. Int. Ed. Engl. 2009, 48, 1740 |
| 113. T. Kramer, F. Lo Monte, S. Göring, G.M. Okala Amombo, B. Schmidt, Small molecule kinase inhibitors for LRRK2 and their application to Parkinson´s disease models. ACS Chemical Neuroscience 2012, 3(3), 151-160 |
| 114. T. Kramer, B. Schmidt, F. Lo Monte, International Journal of Alzheimer's Disease, Small-molecule inhibitors of GSK3 – Structural insights and their application to Alzheimer´s disease models, 2012, accepted. 31.1.2012 |
| 115. Schulz, S. Göring, B. Schmidt, C. Hopf, LRRK2 Kinase Inhibitors as New Drugs for Parkinson’s Disease? in Emerging Drugs and Target for Parkinson’s Disease, RSC Drug Discovery Series No. 34, 2013, p. 266-293. Ed. Ana Martinez, Carmen Gil. RSC Publishing, Cambridge, UK. ISBN: 978-1-84973-617-6 |
| 116. Schmidt, B. Auf dem Weg zum Aflatoxin M1 und verwandten Strukturen durch homogene Katalyse, Dissertation, Universität Hannover 1991 |
| 117. B. Schmidt, V. Neitemeier, D.K. Ehlert, A. Backhaus-Ehlert, 6-Pyridylnicotines and 2,6-Pyridyl-dicarboxylates for Supramolecular Chemistry, no. 008 “Electronic Conference on Heterocyclic Chemistry 98”: H.S. Rzepa, O. Kappe (Eds), Imperial College Press, 1998, ISBN 81-02-3594-1,http://www.ch.ic.ac.uk/ectoc/echet98/pub/008/index.ht |
| 118. Workshop of Young European Bio-Organic Chemists: Ed.: Ludger A. Wessjohann, Koeln : Prosciencia-Verl.-Buchh., Philipp, 1998, ISBN 3-932265-01-7 |
| 119. Schmidt, B. Biomimetika – Ihre Synthese durch Übergangsmetall-katalysierte Schlüssel-Reaktionen und ihre Anwendung; Habilitationsschrift, Universität Hannover 1998 |
| 120. B. Schmidt, A. Siegler. Aspartic Proteases involved in Alzheimer's disease, Highlights in Bioorganic Chemistry, ed. C. Schmuck, H. Wennemers. ISBN 3-527-30656-0, Wiley-VCH, Weinheim 2004 |
| 121. Schmidt, B. Larbig, G. ChemBioChem, 2004, 5, |
| 122. Schmidt, B. ChemBioChem, 2004, 5, 1153-1154. |
| 123. Schmidt, B. ChemBioChem, 2005, 6, 760-761 |
| 124. B. Schmidt, Proteins – Structure and Function, ChemBioChem, 2006, 7 (4), 702-703 |
| 125. B. Schmidt, The Adrenergic Receptors for the 21st Century, ChemMedChem, 2006, 1 (8), 904 |
| 126. S. Baumann, N. Höttecke, B. Schmidt, gamma-secretase as a target for AD, in Medicinal Chemistry of Alzheimer’s Disease, ed. A. Martinez, Research Signpost, 2008 |
| 127. N. Höttecke, S. Baumann, A. Taghavi, H.A. Braun, B. Schmidt, Drug Development and Diagnostics for Alzheimer's Disease Up to 2008, in: Frontiers in Medicinal Chemistry Vol. 4, Ed.: Atta-ur-Rahman, A.B. Reitz, 2009, pp. 730-766, Bentham Books 2009, ISBN 90-77527-07-9 |