 Zhiyong Wang
Zhiyong Wang
WANG Zhiyong(汪志勇)
 Ph.D., University of Science and Technology of China (USTC) (1992); M.S., USTC (1989); B.S., Anhui Normal University (1982).
Ph.D., University of Science and Technology of China (USTC) (1992); M.S., USTC (1989); B.S., Anhui Normal University (1982).
 Professor of Chemistry
Professor of Chemistry
Department of Chemistry
School of Chemistry and Materials Science
University of Science and Technology of China
Hefei, Anhui 230026, P. R. China
Tel: 86-551-63603185
Fax: 86-551-63603185
E-mail: zwang3@ustc.edu.cn
Personal Homepage:
http://staff.ustc.edu.cn/~zwang3/default.htm
RESEARCH INTERESTS
Research
in our group will focus on the general areas of reaction development
and chemical synthesis. Our studies will be driven by the discovery of
new and useful catalysts. By virtue of the developed organic reactions
various organic ligands are synthesized and used as probes in biological
progress. Brief summaries of three research directions illustrating
these objectives are shown below:
1) The preparation of heterogeneous catalysts;
2) The theoretical calculation for the mechanism of organic reactions;
The application of organic ligands as probes or inhibitors to explore the molecular mechanism of HIV transcription.
PUBLICATIONS
http://www.researcherid.com/rid/F-7955-2010
| WANG Zhiyong, Professor |
|
 | Name: | Zhiyong Wang(汪志勇) |
| Born: | June, 1962, Anhui, P. R. China |
| Address: | Department of Chemistry, University of Science and Technology of China, 230026 Hefei, P. R. China |
| Tel: | 86-551-63603185 |
| Fax: | 86-551-63603185 |
| E-mail: | zwang3@ustc.edu.cn |
EDUCATION AND RESEARCH EXPERIENCE
| 1978-1982 | B.S., Anhui Normal University |
| 1982-1986 | Lecturer, South Anhui Agricultural College, China |
| 1986-1989 | M.S., University of Science and Technology of China |
| 1989-1992 | Ph.D., University of Science and Technology of China |
| 1992-1997 | Lecturer, Associate Professor, University of Science and Technology of China |
| 1997-1999 | Research Fellow, Tulane University & Brandeis University |
| 1999-Now | Professor of Chemistry, University of Science and Technology of China |
RESEARCH INTERESTS
| 1) | Organic reactions in aqueous media and development of synthetic methodology; |
| 2) | Supramolecular assembly under the control of organic ligands; |
| 3) | Drug design on the base of PCAF bromodomain. |
CURRENT RESEARCH PROJECTS
| 1) | Organic
reactions in water mediated by nano-metals and its application in
asymmetric synthesis, National Natural Science Foundation (2004-2006) |
| 2) | Crystal Engineering under control of organic ligands, Foundation from Education Department of Anhui Province (2003-2005) |
REPRESENTATIVE PUBLICATIONS
| 1) | C-F. Pan, M. Meze, S. Mujtaba, M. Muller, L. Zeng, J-M. Li, Z-Y. Wang,* M-M. Zhou*
“Structure-Guided Optimization of Small Molecules Selectively
Inhibiting HIV-1 Tat and PCAF Association” J. Med. Chem., 2007, 50, 2285 |
| 2) | Y. Xie, Z-P. Yu, X-Y. Huang, Z-Y. Wang,* L-W. Niu, M-K. Teng, J. Li
“Rational Design on the MOFs Constructed from modified Aromatic Amino Acids”
Chem. Eur. J., 2007, 13, 9399 |
| 3) | Z-H.
Zhang, C-F. Pan, Z-Y. Wang* “Synthesis of chromanones: a novel
palladium-catalyzed Wacker-type oxidative cyclization involving
1,5-hydride alkyl to palladium migration” Chem. Commun, 2007, 4686 |
| 4) | Y.
Xie, Y. Yan, H-H. Wu, G-P. Yong, Y. Cui, Z-Y. Wang*, L. Pan, J. Li
“Homochiral Metal-organic Coordination Networks from L-Tryptophan”
Inorg. Chim. Acta., 2007, 360,1669 |
| 5) | Y.
Xie, H-H. Wu, G-P. Yong,, Z-Y. Wang*, R. Fan , R-P. Li, G-Q. Pan, Y-C.
Tian, L-S. Sheng, L. Pan, J. Li “Synthesis, Crystal Structure,
Spectroscopic and Magnetic Properties of Two Cobalt Molecules
Constructed from Histidine” J. Mol. Struct., 2007, 833, 88 |
| 6) | Z-H.
Zhang, Z-Y. Wang* “Diatomite-Supported Pd Nanoparticles: An Efficient
Catalyst for Heck and Suzuki Reactions” J. Org. Chem., 2006, 71, 7485 |
| 7) | Z-H.
Zhang, Z-G. Zha, C-S. Gan, C-F. Pan, Y-Q. Zhou, Z-Y. Wang*, M-M. Zhou*
“Catalysis and Regioselectivity of the Aqueous Heck Reaction by Pd(0)
Nanoparticles under Ultrasonic Irradiation”
J. Org. Chem., 2006, 71, 4339 |
|
|
 Scheme 1
Control experiments.
Scheme 1
Control experiments.
The
ubiquitous oxazoles have attracted more and more attention in both
industrial and academic fields for decades. This interest arises from
the fact that a variety of natural and synthetic compounds which contain
the oxazole substructure exhibit significant biological activities and
antiviral properties. Although various synthetic methodologies for
synthesis of oxazols have been reported, the development of milder and
more general procedure to access oxazoles is still desirable.
Initially, compound
A, formed by the substitution reaction of
1a with
2a, which can be transformed following two pathways: (a) I
+, generated by the oxidation of iodine, could oxidize
A to radical intermediate
B, which eliminates one molecular of CO
2 to generate radical
C, which is further oxidized to imine
Dor its isomer
E. Subsequently,
F is obtained by intramolecular nucleophilic addition of
E. Finally, the desired product (
3a) is given by deprotonation and oxidation of
F; (b)
G is formed from the oxidation of
A. Then
3a is obtained through
H, I, J, K following a process similar to path a.
 Scheme 2
Plausible mechanism.
Scheme 2
Plausible mechanism.
General procedure for the synthesis of polysubstituted oxazoles
1a (105.8 mg, 0.7 mmol),
2a (99.5 mg, 0.5 mmol), I
2
(50.8 mg, 0.2 mmol), DMA (2 mL) and TBHP (70% aqueous solution, 1 mmol)
were placed in a tube (10 mL) and sealed with a thin film. Then the
reaction mixture was stirred at 25°C for 4 h, heated up to 60°C and
stirred at this temperature for another 4 h. After that, the resulting
mixture was cooled to the room temperature, diluted with water,
extracted with ethyl acetate. The organic phase was washed with
saturation sodium chloride solution, dried and filtrated. The solvent
was evaporated under reduced pressure and the residue was purified by
silica gel column separation (petroleum ether:ethyl acetate = 10:1) to
give
3a(154.7 mg, 70%) as light yellow solid, mp = 70–72°C.
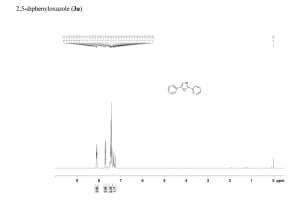
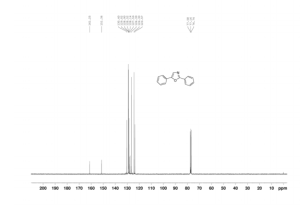
2,5-diphenyloxazole (3a) [1]
Synthesized
according to typical procedure and purified by column chromatography
(petroleum ether:ethyl acetate = 10:1) to give light yellow solid (154.7
mg, 70%), mp = 70-72 °C.
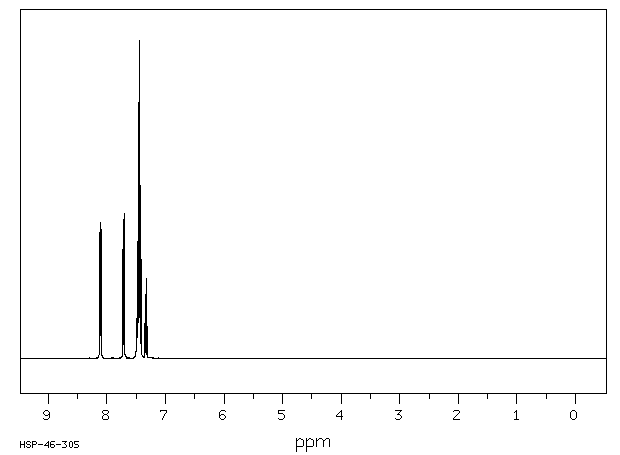
1H NMR (300 MHz, CDCl3): δ 8.12-8.09 (m, 2 H), 7.72-7.69 (m, 2 H), 7.50-7.40 (m, 6 H), 7.35-7.24 (m, 1 H).
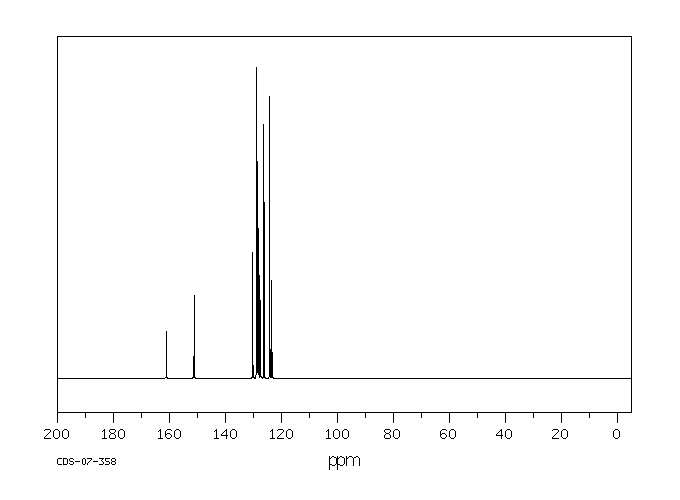
13C NMR (75 MHz, CDCl3): δ 161.3, 151.4, 130.4, 129.0, 128.9, 128.5, 128.1, 127.6, 126.4, 124.3, 123.6.
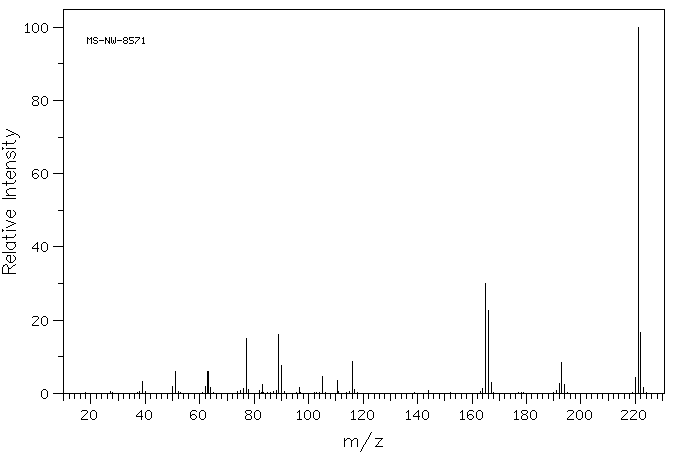
HRMS (APCI-FTMS) m/z: [M + H]+ calcd for C15H12NO: 222.0913, Found: 222.0911.

The scope of the reaction. Standard conditions: 0.7 mmol of amino acids (1a-1h), 0.5 mmol of2a-2j, 0.1 mmol of I2,
1 mmol of TBHP, 2 mL of DMA, were stirred at 25°C for 4 h then slowly
raised to 60°C for 4 h. Catalysts amount and isolated yields were based
on 2.
Metal-free synthesis of polysubstituted oxazoles via a decarboxylative cyclization from primary α-amino acids
Yunfeng Li, Fengfeng Guo, Zhenggen Zha and Zhiyong Wang*
 Zhiyong Wang
Zhiyong Wang
Department
of Chemistry, Hefei National Laboratory for Physical Sciences at
Microscale, CAS Key Laboratory of Soft Matter Chemistry, University of
Science and Technology of China, Hefei, Anhui 230026, P. R. China
ADDITIONAL SPECTRAL DATA FROM NET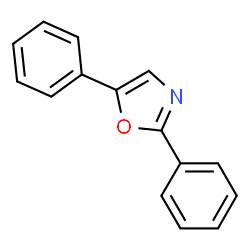
Hefei, Anhui China









////Metal-free, Synthesis, Oxazoles, Oxidation, Decarboxylative cyclization, α-amino acids















 Zhiyong Wang
Zhiyong Wang



No comments:
Post a Comment