Professor Faiza M. Al-KharafiDepartment of Chemistry
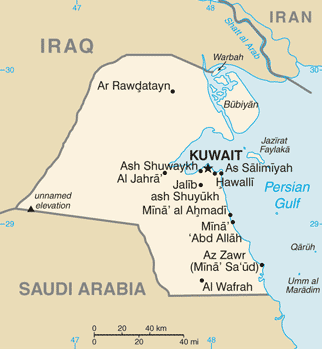
Kuwait University
State of Kuwait
Tel: 00965- 4811188 Ext. 7453
Email : chesc@kuc01.kuniv.edu.kw

Faiza Al-Kharafi (Arabic: فايزة الخرافي Fāyzah al-Kharāfī; born 1946) is a Kuwaiti chemist and academic. She was the president ofKuwait University from 1993 to 2002 and was the first woman to head a major university in the Middle East.[1] She is the vice president of the World Academy of Sciences.
| Faiza Mohammed Al-Kharafi | |
|---|---|
| Born | 1946 (age 68–69) Kuwait |
| Fields | Electrochemistry, corrosion engineering |
| Alma mater | Ain Shams University Kuwait University |
Early life and education
Faiza Al-Kharafi was born to a wealthy family in Kuwait in 1946. She attended Al Merkab High School. She received her BSc from Ain Shams University in Cairo in 1967. She then attended Kuwait University where she founded the Corrosion and Electrochemistry Research Laboratory. She received her master's in 1972 and her PhD in 1975.[2]
Career
Al-Kharafi worked in Kuwait University's Department of Chemistry from 1975 to 1981. In 1984 she became chair of the department and served as Dean of the Faculty of Science from 1986 to 1989.[2] She became a professor of chemistry at Kuwait University in 1987.[3] On 5 July 1993, Emir Jaber Al-Ahmad Al-Jaber Al-Sabah issued a decree appointing Al-Kharafi as rector of the University,[4] and she became the first woman to head a major university in the Middle East.[1] She served as president from 1993 to 2002 where she oversaw a staff of 1,500.
Al-Kharafi has studied the impact of corrosion on engine cooling systems, distillation units for crude oil, and high temperature geothermal brines. As an electrochemist, she has studied the electrochemical behavior of aluminum, copper, platinum, niobium, vanadium, cadmium, brass, cobalt, and low carbon steel.[5] She collaborated on the discovery of a class of molybdenum-based catalysts that improve gasoline octane without benzene by-products.[6]
She joined the Board of the United Nations University in 1998.[7] Following the passage of women's suffrage in Kuwait in 2005, she said "[w]hen we have political rights, we can express our opinion and vote for the correct person... This gives us the chance to express our ideas."[1] In 2006, she helped to found the American Bilingual School in Kuwait. She is the vice president of The World Academy of Sciences. She is a board member of the Kuwait Foundation for the Advancement Sciences.
Works
- Al-Kharafi, Faiza (1983). Al Saratan Aw Al Khelyat Al Moutamarida [Cancer or Rebellious Cells].
- Al-Kharafi, Faiza (1986). Al Hareb Al Kimaeya [Chemical War].
- Abdullah, Aboubakr M.; Al-Kharafi, Faiza M.; Ateya, Badr G. (May 2006). "Intergranular corrosion of copper in the presence of benzotriazole". Scripta Materialia 54 (9): 1673–1677. doi:10.1016/j.scriptamat.2006.01.014.
- Makhseed, Saad; Al-Kharafi, Faiza; Samuel, Jacob; Ateya, Badr (25 April 2009). "Catalytic oxidation of sulphide ions using a novel microporous cobalt phthalocyanine network polymer in aqueous solution". Catalysis Communications 10 (9): 1284–1287. doi:10.1016/j.catcom.2009.01.034.
Awards and honours
Forbes magazine named her as one of "The 100 Most Powerful Women – Women To Watch In The Middle East" in 2005.[1] She received the Kuwait Prize in Applied Sciences in 2006. The Council for Gulf Relations named her Top Gulf Woman of the Year in 2008.[8] In 2011 she was the recipient of the L'Oréal-UNESCO Award for Women in Science for her work on corrosion.[9]
Personal life
Al-Kharafi is married to Ali Mohammed Thanian Al-Ghanim and has five sons and ten grandchildren. Of her sons, Marzouq Al-Ghanim is the current speaker of Kuwait National Assembly. She spends her Summers at Lake Geneva, Switzerland. Her brothers are Jassem Al-Kharafi, former speaker of the Kuwaiti National Assembly, and the late Nasser Al-Kharafi. She shares in the family fortune from M. A. Kharafi & Sons.[2][8]
References
- ^ a b c d "Middle Eastern Women To Watch". Forbes. 26 July 2005.
- ^ a b c "Fayza Al Khorafi". Who's Who Amongst Arab Women. Retrieved 20 June 2013.
- ^ O'Shea, Maria (1999). Kuwait (2nd ed.). New York: Marshall Cavendish Corp. p. 61. ISBN 978-0-7614-0871-0.
- ^ "This day of Kuwait's history". Kuwait News Agency. 5 July 2009.
- ^ "Faiza al-Kharafi". Kuwait–MIT Center. Retrieved 20 June 2013.
- ^ "Faiza Al-Kharafi (Αφρική και Αραβικές Χώρες)". Eleftherotypia. 3 March 2011.
- ^ "This day in Kuwait's history". Kuwait News Agency. 1 March 2008.
- ^ a b Farag, Talaat I. (July 2008). "Dr. Faiza Al-Khorafi, PhD". The Ambassadors Online Magazine.
- "Outstanding women scientists to receive 2011 L’ORÉAL-UNESCO Awards (3 March) and Fellowships (2 March)". UNESCO. 25 February 2011.
Further reading
- "Kuwait University leader wants students who can adapt to change". Dallas Morning News. Reuters. 11 December 1993.
- "Kuwait Educator Sees a Need to Adapt College Curriculum to Changing World". Chicago Sun-Times. Reuters. 11 January 1994.
- Bollag, Burton. "A female president, the Arab world's first, guides the restoration of Kuwait U." The Chronicle of Higher Education 40.24 (1994): A45. Biography in Context. Web. 20 June 2013.
External links




/////







 Zhiyong Wang
Zhiyong Wang